Page 776 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 776
759
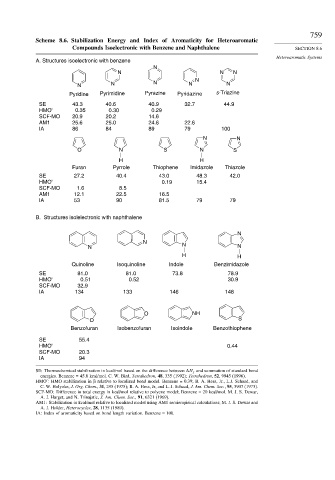
Scheme 8.6. Stabilization Energy and Index of Aromaticity for Heteroaromatic
Compounds Isoelectronic with Benzene and Naphthalene SECTION 8.6
Heteroaromatic Systems
A. Structures isoelectronic with benzene
N
N N N
N
N
N N N N
Pyridine Pyrimidine Pyrazine Pyridazine s-Triazine
SE 43.3 40.6 40.9 32.7 44.9
HMO' 0.35 0.30 0.29
SCF-MO 20.9 20.2 14.6
AM1 25.6 25.0 24.6 22.6
IA 86 84 89 79 100
N N
O N S N S
H H
Furan Pyrrole Thiophene Imidazole Thiazole
SE 27.2 40.4 43.0 48.3 42.0
HMO' 0.19 15.4
SCF-MO 1.6 8.5
AM1 12.1 22.5 16.5
IA 53 90 81.5 79 79
B. Structures isolelectronic with naphthalene
N
N
N N N
H H
Quinoline Isoquinoline Indole Benzimidazole
SE 81.0 81.0 73.8 78.9
HMO' 0.51 0.52 30.9
SCF-MO 32.9
IA 134 133 146 148
O NH
O S
Benzofuran Isobenzofuran Isoindole Benzothiophene
SE 55.4
HMO' 0.44
SCF-MO 20.3
IA 94
SE: Thermochemical stabilization in kcal/mol based on the difference between H f and summation of standard bond
energies. Benzene = 45.8 kcal/mol. C. W. Bird, Tetrahedron, 48, 335 (1992); Tetrahedron, 52, 9945 (1996).
HMO : HMO stabilization in relative to localized bond model. Benzene = 0.39; B. A. Hess, Jr., L.J. Schaad, and
C. W. Holyoke, J. Org. Chem., 31, 295 (1975); B. A. Hess, Jr, and L. J. Schaad, J. Am. Chem. Soc., 95, 3907 (1973).
SCF-MO: Difference in total energy in kcal/mol relative to polyene model; Benzene = 20 kcal/mol. M. J. S. Dewar,
A. J. Harget, and N. Trinajstic, J. Am. Chem. Soc., 91, 6321 (1969).
AM1: Stabilization in kcal/mol relative to localized model using AM1 semiempirical calculations; M. J. S. Dewar and
A. J. Holder, Heterocycles, 28, 1135 (1989).
IA: Index of aromaticity based on bond length variation. Benzene = 100.