Page 779 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 779
762 c. What are some of the experimental and computational criteria that could be
applied to assess aromaticity or antiaromaticity? Cite at least three such probes
CHAPTER 8 and indicate the nature of the observation and interpretation.
Aromaticity
8.6. Using the empirically chosen energy equivalents for bond types given on p. 748
and a standard compilation of HMO calculations, determine the resonance
energies of the following molecules by the Hess-Schaad procedure (p. 747). Do
you find any discrepancies between the predicted and observed properties, as
described in Section 8.5?
(a) (b) (c) (d) (e)
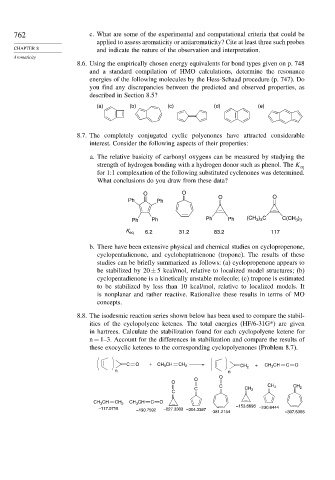
8.7. The completely conjugated cyclic polyenones have attracted considerable
interest. Consider the following aspects of their properties:
a. The relative basicity of carbonyl oxygens can be measured by studying the
strength of hydrogen bonding with a hydrogen donor such as phenol. The K eq
for 1:1 complexation of the following substituted cyclenones was determined.
What conclusions do you draw from these data?
O O
O O
Ph Ph
3 3
Ph Ph Ph Ph (CH ) C C(CH )
3 3
K eq 6.2 31.2 83.2 117
b. There have been extensive physical and chemical studies on cyclopropenone,
cyclopentadienone, and cycloheptatrienone (tropone). The results of these
studies can be briefly summarized as follows: (a) cyclopropenone appears to
be stabilized by 20 ± 5 kcal/mol, relative to localized model structures; (b)
cyclopentadienone is a kinetically unstable molecule; (c) tropone is estimated
to be stabilized by less than 10 kcal/mol, relative to localized models. It
is nonplanar and rather reactive. Rationalize these results in terms of MO
concepts.
8.8. The isodesmic reaction series shown below has been used to compare the stabil-
ities of the cyclopolyene ketenes. The total energies (HF/6-31G*) are given
in hartrees. Calculate the stabilization found for each cyclopolyene ketene for
n = 1–3. Account for the differences in stabilization and compare the results of
these exocyclic ketenes to the corresponding cyclopolyenones (Problem 8.7).
C O + CH 3 CH CH 2 + CH 3 CH C O
CH 2
n n
O
O
O
C CH 2 CH 2
C CH 2
C
CH 3 CH CH 2 CH 3 CH C O
–153.6698
–117.0715 –227.3303 –304.3387 –230.6444
–190.7592
-381.2154 –307.5368