Page 774 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 774
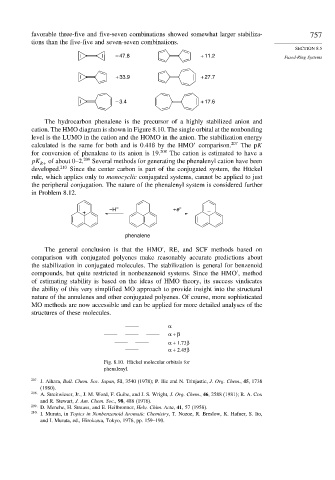
favorable three-five and five-seven combinations showed somewhat larger stabiliza- 757
tions than the five-five and seven-seven combinations.
SECTION 8.5
– 47.8 + 11.2 Fused-Ring Systems
+ 33.9 + 27.7
– 3.4 + 17.6
The hydrocarbon phenalene is the precursor of a highly stabilized anion and
cation. The HMO diagram is shown in Figure 8.10. The single orbital at the nonbonding
level is the LUMO in the cation and the HOMO in the anion. The stabilization energy
calculated is the same for both and is 0.41ß by the HMO’ comparison. 207 The pK
for conversion of phenalene to its anion is 19. 208 The cation is estimated to have a
pK of about 0–2. 209 Several methods for generating the phenalenyl cation have been
R+
developed. 210 Since the center carbon is part of the conjugated system, the Hückel
rule, which applies only to monocyclic conjugated systems, cannot be applied to just
the peripheral conjugation. The nature of the phenalenyl system is considered further
in Problem 8.12.
–H – +e –
+ –
phenalene
The general conclusion is that the HMO , RE, and SCF methods based on
comparison with conjugated polyenes make reasonably accurate predictions about
the stabilization in conjugated molecules. The stabilization is general for benzenoid
compounds, but quite restricted in nonbenzenoid systems. Since the HMO , method
of estimating stability is based on the ideas of HMO theory, its success vindicates
the ability of this very simplified MO approach to provide insight into the structural
nature of the annulenes and other conjugated polyenes. Of course, more sophisticated
MO methods are now accessible and can be applied for more detailed analyses of the
structures of these molecules.
α
α + β
α + 1.73β
α + 2.45β
Fig. 8.10. Hückel molecular orbitals for
phenalenyl.
207 J. Aihara, Bull. Chem. Soc. Japan, 51, 3540 (1978); P. Ilic and N. Trinjastic, J. Org. Chem., 45, 1738
(1980).
208
A. Streitwieser, Jr., J. M. Word, F. Guibe, and J. S. Wright, J. Org. Chem., 46, 2588 (1981); R. A. Cox
and R. Stewart, J. Am. Chem. Soc., 98, 488 (1976).
209 D. Menche, H. Strauss, and E. Heilbronner, Helv. Chim. Acta, 41, 57 (1958).
210
I. Murata, in Topics in Nonbenzenoid Aromatic Chemistry, T. Nozoe, R. Breslow, K. Hafner, S. Ito,
and I. Murata, ed., Hirokawa, Tokyo, 1976, pp. 159–190.