Page 777 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 777
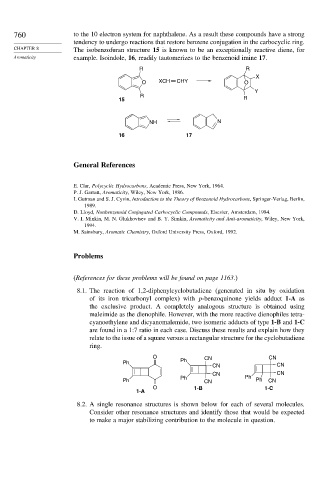
760 to the 10 electron system for naphthalene. As a result these compounds have a strong
tendency to undergo reactions that restore benzene conjugation in the carbocyclic ring.
CHAPTER 8 The isobenzofuran structure 15 is known to be an exceptionally reactive diene, for
Aromaticity example. Isoindole, 16, readily tautomerizes to the benzenoid imine 17.
R R
X
O XCH CHY O
Y
R
15 R
NH N
16 17
General References
E. Clar, Polycyclic Hydrocarbons, Academic Press, New York, 1964.
P. J. Garratt, Aromaticity, Wiley, New York, 1986.
I. Gutman and S. J. Cyvin, Introduction to the Theory of Benzenoid Hydrocarbons, Springer-Verlag, Berlin,
1989.
D. Lloyd, Nonbenzenoid Conjugated Carbocyclic Compounds, Elsevier, Amsterdam, 1984.
V. I. Minkin, M. N. Glukhovtsev and B. Y. Simkin, Aromaticity and Anti-aromaticity, Wiley, New York,
1994.
M. Sainsbury, Aromatic Chemistry, Oxford University Press, Oxford, 1992.
Problems
(References for these problems will be found on page 1163.)
8.1. The reaction of 1,2-diphenylcyclobutadiene (generated in situ by oxidation
of its iron tricarbonyl complex) with p-benzoquinone yields adduct 1-A as
the exclusive product. A completely analogous structure is obtained using
maleimide as the dienophile. However, with the more reactive dienophiles tetra-
cyanoethylene and dicyanomalemide, two isomeric adducts of type 1-B and 1-C
are found in a 1:7 ratio in each case. Discuss these results and explain how they
relate to the issue of a square versus a rectangular structure for the cyclobutadiene
ring.
O CN CN
Ph Ph
CN CN
CN Ph CN
Ph Ph CN Ph CN
O 1-B 1-C
1-A
8.2. A single resonance structures is shown below for each of several molecules.
Consider other resonance structures and identify those that would be expected
to make a major stabilizing contribution to the molecule in question.