Page 773 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 773
756 Because the five-membered ring is a substituted cyclopentadienide anion in some
dipolar resonance structures, it might be expected that exocyclic groups that could
CHAPTER 8 strongly stabilize a positive charge might lead to a larger contribution from dipolar
Aromaticity structures and enhanced stability. Structures 13 and 14 are cases in which a large
dipolar contribution would be feasible.
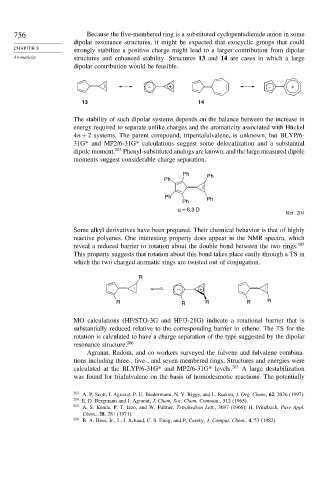
– + – +
13 14
The stability of such dipolar systems depends on the balance between the increase in
energy required to separate unlike charges and the aromaticity associated with Hückel
4n + 2 systems. The parent compound, tripentafulvalene, is unknown, but BLYP/6-
31G* and MP2/6-31G* calculations suggest some delocalization and a substantial
dipole moment. 203 Phenyl-substituted analogs are known, and the large measured dipole
moments suggest considerable charge separation.
Ph Ph
Ph
Ph Ph
Ph
μ = 6.3 D
Ref. 204
Some alkyl derivatives have been prepared. Their chemical behavior is that of highly
reactive polyenes. One interesting property does appear in the NMR spectra, which
reveal a reduced barrier to rotation about the double bond between the two rings. 205
This property suggests that rotation about this bond takes place easily through a TS in
which the two charged aromatic rings are twisted out of conjugation.
R
– +
R R R R R
MO calculations (HF/STO-3G and HF/3-21G) indicate a rotational barrier that is
substantially reduced relative to the corresponding barrier in ethene. The TS for the
rotation is calculated to have a charge separation of the type suggested by the dipolar
resonance structure. 206
Agranat, Radom, and co-workers surveyed the fulvene and fulvalene combina-
tions including three-, five-, and seven-membered rings. Structures and energies were
calculated at the BLYP/6-31G* and MP2/6-31G* levels. 203 A large destabilization
was found for triafulvalene on the basis of homodesmotic reactions. The potentially
203
A. P. Scott, I. Agranat, P. U. Biedermann, N. V. Riggs, and L. Radom, J. Org. Chem., 62, 2026 (1997).
204 E. D. Bergmann and I. Agranat, J. Chem. Soc. Chem. Commun., 512 (1965).
205 A. S. Kende, P. T. Izzo, and W. Fulmer, Tetrahedron Lett., 3697 (1966); H. Prinzbach, Pure Appl.
Chem., 28, 281 (1971).
206
B. A. Hess, Jr., L. J. Schaad, C. S. Ewig, and P. Carsky, J. Comput. Chem., 4, 53 (1982).