Page 768 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 768
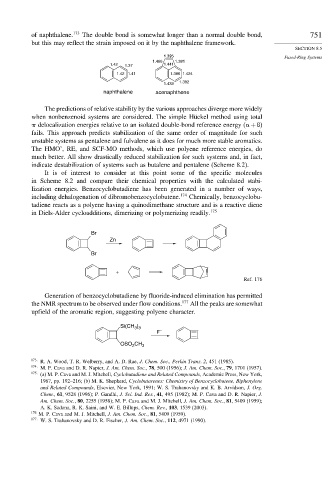
of naphthalene. 173 The double bond is somewhat longer than a normal double bond, 751
but this may reflect the strain imposed on it by the naphthalene framework.
SECTION 8.5
1.395 Fused-Ring Systems
1.466 1.381
1.42 1.37 1.441
1.42 1.41 1.386 1.424
1.433 1.382
naphthalene acenaphthene
The predictions of relative stability by the various approaches diverge more widely
when nonbenzenoid systems are considered. The simple Hückel method using total
delocalization energies relative to an isolated double-bond reference energy ( +ß)
fails. This approach predicts stabilization of the same order of magnitude for such
unstable systems as pentalene and fulvalene as it does for much more stable aromatics.
The HMO’, RE, and SCF-MO methods, which use polyene reference energies, do
much better. All show drastically reduced stabilization for such systems and, in fact,
indicate destabilization of systems such as butalene and pentalene (Scheme 8.2).
It is of interest to consider at this point some of the specific molecules
in Scheme 8.2 and compare their chemical properties with the calculated stabi-
lization energies. Benzocyclobutadiene has been generated in a number of ways,
including dehalogenation of dibromobenzocyclobutene. 174 Chemically, benzocyclobu-
tadiene reacts as a polyene having a quinodimethane structure and is a reactive diene
in Diels-Alder cycloadditions, dimerizing or polymerizing readily. 175
Br
Zn
Br
+
Ref. 176
Generation of benzocyclobutadiene by fluoride-induced elimination has permitted
the NMR spectrum to be observed under flow conditions. 177 All the peaks are somewhat
upfield of the aromatic region, suggesting polyene character.
Si(CH )
3 3
F –
OSO CH 3
2
173 R. A. Wood, T. R. Welberry, and A. D. Rae, J. Chem. Soc., Perkin Trans. 2, 451 (1985).
174
M. P. Cava and D. R. Napier, J. Am. Chem. Soc., 78, 500 (1956); J. Am. Chem. Soc., 79, 1701 (1957).
175
(a) M. P. Cava and M. J. Mitchell, Cyclobutadiene and Related Compounds, Academic Press, New York,
1967, pp. 192–216; (b) M. K. Shepherd, Cyclobutarenes: Chemistry of Benzocyclobutene, Biphenylene
and Related Compounds, Elsevier, New York, 1991; W. S. Trahanovsky and K. B. Arvidson, J. Org.
Chem., 61, 9528 (1996); P. Gandhi, J. Sci. Ind. Res., 41, 495 (1982); M. P. Cava and D. R. Napier, J.
Am. Chem. Soc., 80, 2255 (1958); M. P. Cava and M. J. Mitchell, J. Am. Chem. Soc., 81, 5409 (1959);
A. K. Sadana, R. K. Saini, and W. E. Billups, Chem. Rev., 103, 1539 (2003).
176 M. P. Cava and M. J. Mitchell, J. Am. Chem. Soc., 81, 5409 (1959).
177
W. S. Trahanovsky and D. R. Fischer, J. Am. Chem. Soc., 112, 4971 (1990).