Page 765 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 765
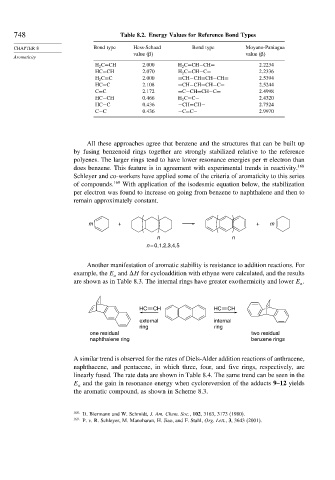
748 Table 8.2. Energy Values for Reference Bond Types
CHAPTER 8 Bond type Hess-Schaad Bond type Moyano-Paniagua
value ( ) value ( )
Aromaticity
H 2 C=CH 2 000 H 2 C=CH−CH= 2 2234
HC=CH 2 070 H 2 C=CH−C= 2 2336
H 2 C=C 2 000 =CH−CH=CH−CH= 2 5394
HC=C 2 108 =CH−CH=CH−C= 2 5244
C=C 2 172 =C−CH=CH−C= 2 4998
HC−CH 0 466 H 2 C=C− 2 4320
HC−C 0 436 −CH=CH− 2 7524
C−C 0 436 −C=C− 2 9970
All these approaches agree that benzene and the structures that can be built up
by fusing benzenoid rings together are strongly stabilized relative to the reference
polyenes. The larger rings tend to have lower resonance energies per electron than
does benzene. This feature is in agreement with experimental trends in reactivity. 168
Schleyer and co-workers have applied some of the criteria of aromaticity to this series
of compounds. 169 With application of the isodesmic equation below, the stabilization
per electron was found to increase on going from benzene to naphthalene and then to
remain approximately constant.
m + + m
n n
n = 0,1,2,3,4,5
Another manifestation of aromatic stability is resistance to addition reactions. For
example, the E and H for cycloaddition with ethyne were calculated, and the results
a
are shown as in Table 8.3. The internal rings have greater exothermicity and lower E
a
HC CH HC CH
external internal
ring ring
one residual two residual
naphthalene ring benzene rings
A similar trend is observed for the rates of Diels-Alder addition reactions of anthracene,
naphthacene, and pentacene, in which three, four, and five rings, respectively, are
linearly fused. The rate data are shown in Table 8.4. The same trend can be seen in the
E and the gain in resonance energy when cycloreversion of the adducts 9–12 yields
a
the aromatic compound, as shown in Scheme 8.3.
168 D. Biermann and W. Schmidt, J. Am. Chem. Soc., 102, 3163, 3173 (1980).
169
P. v. R. Schleyer, M. Manoharan, H. Jiao, and F. Stahl, Org. Lett., 3, 3643 (2001).