Page 761 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 761
744 the planar structure accounts for most of the difference. 152 A higher estimate of the
homoaromatic stabilization of 13.4 kcal/mol results from a calculation that assigns the
CHAPTER 8 153
difference in strain as 10.1 kcal/mol.
Aromaticity
The cyclobutenyl cation 7 is the homoaromatic analog of the very stable
cyclopropenium cation. This ion can be prepared from 3-acetoxycyclobutene using
“superacid” conditions. 154
H H
HOSO F
2
+
SbF , SO ClF
O CCH 3 5 2
2
–78°C 7
The homoaromatic cyclobutenylium ion is calculated to be 10.3 kcal/mol less stable
than the isomeric methylcyclopropenylium ion, but the barrier for interconversion is
high. 155 The temperature-dependent NMR spectrum of the ion can be analyzed to show
that there is a barrier (8.4 kcal/mol) for the ring flip that interchanges the two hydrogens
of the methylene group. The 13 C-NMR chemical shift is also compatible with the
homoaromatic structure. MO (MP3/6-31G*) calculations are successful in reproducing
the structural and spectroscopic characteristics of the cation and are consistent with
a homoaromatic structure. 156 Analysis of electron density did not find a bond critical
point between C(1) and C(3), but the electron density is equivalent to a bond order of
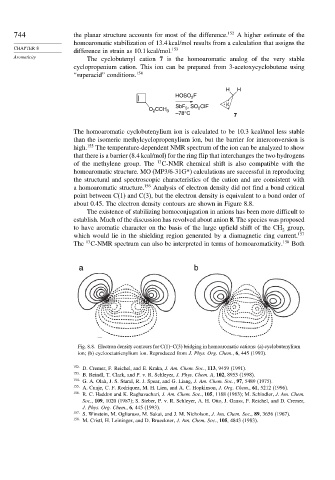
about 0.45. The electron density contours are shown in Figure 8.8.
The existence of stabilizing homoconjugation in anions has been more difficult to
establish. Much of the discussion has revolved about anion 8. The species was proposed
to have aromatic character on the basis of the large upfield shift of the CH group,
2
which would lie in the shielding region generated by a diamagnetic ring current. 157
The 13 C-NMR spectrum can also be interpreted in terms of homoaromaticity. 158 Both
a b
Fig. 8.8. Electron density contours for C(1)–C(3) bridging in homoaromatic cations: (a) cyclobutenylium
ion; (b) cyclooctatrienylium ion. Reproduced from J. Phys. Org. Chem., 6, 445 (1993).
152
D. Cremer, F. Reichel, and E. Kraka, J. Am. Chem. Soc., 113, 9459 (1991).
153 B. Reindl, T. Clark, and P. v. R. Schleyer, J. Phys. Chem. A, 102, 8953 (1998).
154
G. A. Olah, J. S. Staral, R. J. Spear, and G. Liang, J. Am. Chem. Soc., 97, 5489 (1975).
155
A. Cunje, C. F. Rodriquez, M. H. Lien, and A. C. Hopkinson, J. Org. Chem., 61, 5212 (1996).
156 R. C. Haddon and K. Raghavachari, J. Am. Chem. Soc., 105, 1188 (1983); M. Schindler, J. Am. Chem.
Soc., 109, 1020 (1987); S. Sieber, P. v. R. Schleyer, A. H. Otto, J. Gauss, F. Reichel, and D. Cremer,
J. Phys. Org. Chem., 6, 445 (1993).
157 S. Winstein, M. Ogliaruso, M. Sakai, and J. M. Nicholson, J. Am. Chem. Soc., 89, 3656 (1967).
158
M. Cristl, H. Leininger, and D. Brueckner, J. Am. Chem. Soc., 105, 4843 (1983).