Page 762 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 762
C8 745
C1 SECTION 8.5
Fused-Ring Systems
C2
C7
C6 C5 C6
C3
C12 Li
N1 C11
N2
C13
C9
C14
C10
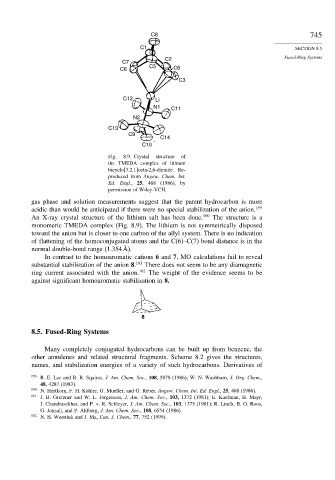
Fig. 8.9. Crystal structure of
the TMEDA complex of lithium
bicyclo[3.2.1]octa-2,6-dienide. Re-
produced from Angew. Chem. Int.
Ed. Engl., 25, 468 (1986), by
permission of Wiley-VCH.
gas phase and solution measurements suggest that the parent hydrocarbon is more
acidic than would be anticipated if there were no special stabilization of the anion. 159
An X-ray crystal structure of the lithium salt has been done. 160 The structure is a
monomeric TMEDA complex (Fig. 8.9). The lithium is not symmetrically disposed
toward the anion but is closer to one carbon of the allyl system. There is no indication
of flattening of the homoconjugated atoms and the C(6)–C(7) bond distance is in the
normal double-bond range (1.354 Å).
In contrast to the homoaromatic cations 6 and 7, MO calculations fail to reveal
substantial stabilization of the anion 8. 161 There does not seem to be any diamagnetic
ring current associated with the anion. 162 The weight of the evidence seems to be
against significant homoaromatic stabilization in 8.
-
8
8.5. Fused-Ring Systems
Many completely conjugated hydrocarbons can be built up from benzene, the
other annulenes and related structural fragments. Scheme 8.2 gives the structures,
names, and stabilization energies of a variety of such hydrocarbons. Derivatives of
159
R. E. Lee and R. R. Squires, J. Am. Chem. Soc., 108, 5078 (1986); W. N. Washburn, J. Org. Chem.,
48, 4287 (1983).
160 N. Hertkorn, F. H. Kohler, G. Mueller, and G. Reber, Angew. Chem. Int. Ed. Engl., 25, 468 (1986).
161
J. B. Grutzner and W. L. Jorgenson, J. Am. Chem. Soc., 103, 1372 (1981); E. Kaufman, H. Mayr,
J. Chandrasekhar, and P. v. R. Schleyer, J. Am. Chem. Soc., 103, 1375 (1981); R. Lindh, B. O. Roos,
G. Jonsall, and P. Ahlberg, J. Am. Chem. Soc., 108, 6554 (1986).
162
N. H. Werstiuk and J. Ma, Can. J. Chem., 77, 752 (1999).