Page 763 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 763
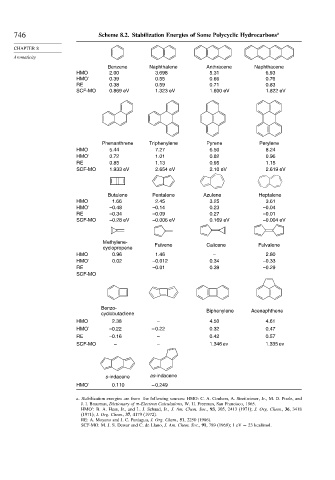
746 Scheme 8.2. Stabilization Energies of Some Polycyclic Hydrocarbons a
CHAPTER 8
Aromaticity
Benzene Naphthalene Anthracene Naphthacene
HMO 2.00 3.698 5.31 6.93
HMO' 0.39 0.55 0.66 0.76
RE 0.38 0.59 0.71 0.83
SCF-MO 0.869 eV 1.323 eV 1.600 eV 1.822 eV
Phenanthrene Triphenylene Pyrene Perylene
HMO 5.44 7.27 6.50 8.24
HMO' 0.72 1.01 0.82 0.96
RE 0.85 1.13 0.95 1.15
SCF-MO 1.933 eV 2.654 eV 2.10 eV 2.619 eV
Butalene Pentalene Azulene Heptalene
HMO 1.66 2.45 3.25 3.61
HMO' –0.48 –0.14 0.23 –0.04
RE –0.34 –0.09 0.27 –0.01
SCF-MO –0.28 eV –0.006 eV 0.169 eV –0.004 eV
Methylene- Fulvene Calicene Fulvalene
cyclopropene
HMO 0.96 1.46 – 2.80
HMO' 0.02 –0.012 0.34 –0.33
RE –0.01 0.39 –0.29
SCF-MO
Benzo-
cyclobutadiene Biphenylene Acenaphthene
HMO 2.38 – 4.50 4.61
HMO' –0.22 – 0.22 0.32 0.47
RE –0.16 – 0.42 0.57
SCF-MO – – 1.346 ev 1.335 ev
s-indacene as-indacene
HMO' 0.110 – 0.249
a. Stabilization energies are from the following sources: HMO: C. A. Coulson, A. Streitwieser, Jr., M. D. Poole, and
J. I. Brauman, Dictionary of -Electron Calculations, W. H. Freeman, San Francisco, 1965.
HMO : B. A. Hess, Jr., and L. J. Schaad, Jr., J. Am. Chem. Soc., 93, 305, 2413 (1971); J. Org. Chem., 36, 3418
(1971); J. Org. Chem., 37, 4179 (1972).
RE: A. Moyano and J. C. Paniagua, J. Org. Chem., 51, 2250 (1986).
SCF-MO: M. J. S. Dewar and C. de Llano, J. Am. Chem. Soc., 91, 789 (1969); 1 eV = 23 kcal/mol.