Page 767 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 767
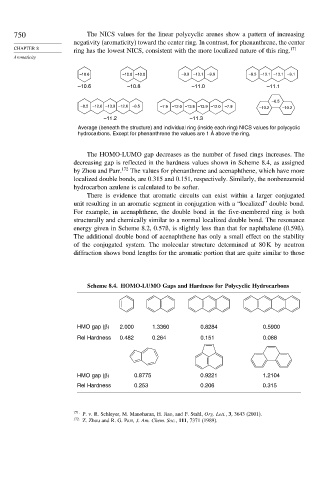
750 The NICS values for the linear polycyclic arenes show a pattern of increasing
negativity (aromaticity) toward the center ring. In contrast, for phenanthrene, the center
CHAPTER 8 171
ring has the lowest NICS, consistent with the more localized nature of this ring.
Aromaticity
–10.6 –10.8 –10.8 –9.9 –13.1 –9.9 –9.3 –13.1 –13.1 –9.1
–10.6 –10.8 –11.0 –11.1
–6.5
–8.5 –12.6 –13.9 –12.6 –8.5 –7.9 –12.0 –13.9 –13.9 –12.0 –7.9 –10.2 –10.2
–11.2 –11.3
Average (beneath the structure) and individual ring (inside each ring) NICS values for polycyclic
hydrocarbons. Except for phenanthrene the values are 1 Å above the ring.
The HOMO-LUMO gap decreases as the number of fused rings increases. The
decreasing gap is reflected in the hardness values shown in Scheme 8.4, as assigned
by Zhou and Parr. 172 The values for phenanthrene and acenaphthene, which have more
localized double bonds, are 0.315 and 0.151, respectively. Similarly, the nonbenzenoid
hydrocarbon azulene is calculated to be softer.
There is evidence that aromatic circuits can exist within a larger conjugated
unit resulting in an aromatic segment in conjugation with a “localized” double bond.
For example, in acenaphthene, the double bond in the five-membered ring is both
structurally and chemically similar to a normal localized double bond. The resonance
energy given in Scheme 8.2, 0.57ß, is slightly less than that for naphthalene (0.59ß).
The additional double bond of acenaphthene has only a small effect on the stability
of the conjugated system. The molecular structure determined at 80K by neutron
diffraction shows bond lengths for the aromatic portion that are quite similar to those
Scheme 8.4. HOMO-LUMO Gaps and Hardness for Polycyclic Hydrocarbons
HMO gap (β) 2.000 1.3360 0.8284 0.5900
Rel Hardness 0.482 0.264 0.151 0.088
HMO gap (β) 0.8775 0.9221 1.2104
Rel Hardness 0.253 0.206 0.315
171 P. v. R. Schleyer, M. Manoharan, H. Jiao, and F. Stahl, Org. Lett., 3, 3643 (2001).
172
Z. Zhou and R. G. Parr, J. Am. Chem. Soc., 111, 7371 (1989).