Page 772 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 772
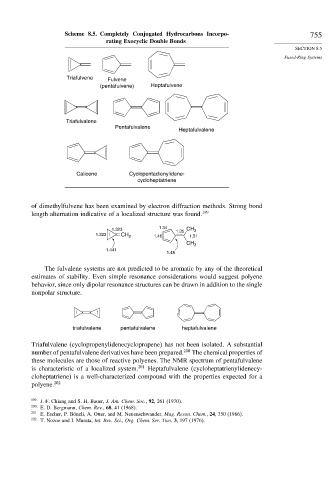
Scheme 8.5. Completely Conjugated Hydrocarbons Incorpo- 755
rating Exocyclic Double Bonds
SECTION 8.5
Fused-Ring Systems
Triafulvene Fulvene
(pentafulvene) Heptafulvene
Triafulvalene
Pentafulvalene
Heptafulvalene
Calicene Cyclopentadienylidene-
cycloheptatriene
of dimethylfulvene has been examined by electron diffraction methods. Strong bond
length alternation indicative of a localized structure was found. 199
1.323 1.34 1.35 CH 3
1.323 CH 2 1.46 1.51
CH 3
1.441
1.48
The fulvalene systems are not predicted to be aromatic by any of the theoretical
estimates of stability. Even simple resonance considerations would suggest polyene
behavior, since only dipolar resonance structures can be drawn in addition to the single
nonpolar structure.
triafulvalene pentafulvalene heptafulvalene
Triafulvalene (cyclopropenylidenecyclopropene) has not been isolated. A substantial
number of pentafulvalene derivatives have been prepared. 200 The chemical properties of
these molecules are those of reactive polyenes. The NMR spectrum of pentafulvalene
is characteristic of a localized system. 201 Heptafulvalene (cycloheptatrienylidenecy-
cloheptatriene) is a well-characterized compound with the properties expected for a
polyene. 202
199
J. F. Chiang and S. H. Bauer, J. Am. Chem. Soc., 92, 261 (1970).
200
E. D. Bergmann, Chem. Rev., 68, 41 (1968).
201 E. Escher, P. Bönzli, A. Otter, and M. Neuenschwander, Mag. Reson. Chem., 24, 350 (1986).
202
T. Nozoe and I. Murata, Int. Rev. Sci., Org. Chem. Ser. Two, 3, 197 (1976).