Page 766 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 766
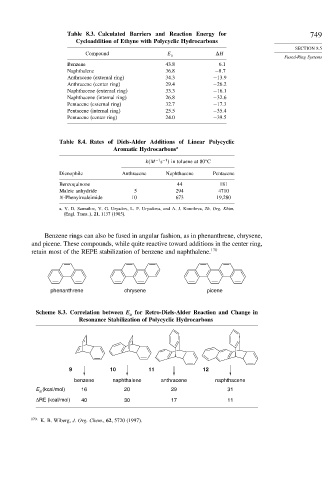
Table 8.3. Calculated Barriers and Reaction Energy for 749
Cycloaddition of Ethyne with Polycyclic Hydrocarbons
SECTION 8.5
Compound E a H
Fused-Ring Systems
Benzene 43.8 6.1
Naphthalene 36.8 −8 7
Anthracene (external ring) 34.3 −13 9
Anthracene (center ring) 29.4 −26 2
Naphthacene (external ring) 33.3 −16 1
Naphthacene (internal ring) 26.8 −32 6
Pentacene (external ring) 32.7 −17 3
Pentacene (internal ring) 25.5 −35 4
Pentacene (center ring) 24.0 −39 5
Table 8.4. Rates of Diels-Alder Additions of Linear Polycyclic
Aromatic Hydrocarbons a
s in toluene at 80 C
k M −1 −1
Dienophile Anthracene Naphthacene Pentacene
Benzoquinone 44 181
Maleic anhydride 5 294 4710
N-Phenylmaleimide 10 673 19,280
a. V. D. Samuilov, V. G. Uryadov, L. F. Uryadova, and A. J. Konolova, Zh. Org. Khim.
(Engl. Trans..), 21, 1137 (1985).
Benzene rings can also be fused in angular fashion, as in phenanthrene, chrysene,
and picene. These compounds, while quite reactive toward additions in the center ring,
retain most of the REPE stabilization of benzene and naphthalene. 170
phenanthrene chrysene picene
Scheme 8.3. Correlation between E for Retro-Diels-Alder Reaction and Change in
a
Resonance Stabilization of Polycyclic Hydrocarbons
9 10 11 12
benzene naphthalene anthracene naphthacene
E a (kcal/mol) 16 20 29 31
ΔRE (kcal/mol) 40 30 17 11
170
K. B. Wiberg, J. Org. Chem., 62, 5720 (1997).