Page 758 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 758
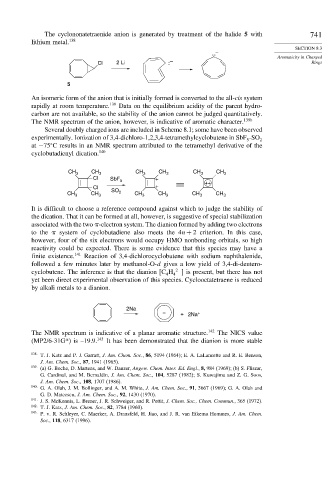
The cyclononatetraenide anion is generated by treatment of the halide 5 with 741
lithium metal. 138
SECTION 8.3
–
Aromaticity in Charged
Cl 2 Li – Rings
5
An isomeric form of the anion that is initially formed is converted to the all-cis system
rapidly at room temperature. 139 Data on the equilibrium acidity of the parent hydro-
carbon are not available, so the stability of the anion cannot be judged quantitatively.
The NMR spectrum of the anion, however, is indicative of aromatic character. 139b
Several doubly charged ions are included in Scheme 8.1; some have been observed
experimentally. Ionization of 3,4-dichloro-1,2,3,4-tetramethylcyclobutene in SbF -SO
5 2
at −75 C results in an NMR spectrum attributed to the tetramethyl derivative of the
cyclobutadienyl dication. 140
CH 3 CH 3 CH 3 CH 3 CH 3 CH 3
Cl SbF 5 + ++
Cl SO +
CH 3 CH 3 2 CH 3 CH 3 CH 3 CH 3
It is difficult to choose a reference compound against which to judge the stability of
the dication. That it can be formed at all, however, is suggestive of special stabilization
associated with the two -electron system. The dianion formed by adding two electrons
to the system of cyclobutadiene also meets the 4n + 2 criterion. In this case,
however, four of the six electrons would occupy HMO nonbonding orbitals, so high
reactivity could be expected. There is some evidence that this species may have a
finite existence. 141 Reaction of 3,4-dichlorocyclobutene with sodium naphthalenide,
followed a few minutes later by methanol-O-d gives a low yield of 3,4-di-deutero-
cyclobutene. The inference is that the dianion [C H 4 2− ] is present, but there has not
4
yet been direct experimental observation of this species. Cyclooctatetraene is reduced
by alkali metals to a dianion.
2Na
= + 2Na +
The NMR spectrum is indicative of a planar aromatic structure. 142 The NICS value
(MP2/6-31G*) is –19.9. 143 It has been demonstrated that the dianion is more stable
138
T. J. Katz and P. J. Garratt, J. Am. Chem. Soc., 86, 5194 (1964); E. A. LaLancette and R. E. Benson,
J. Am. Chem. Soc., 87, 1941 (1965).
139
(a) G. Boche, D. Martens, and W. Danzer, Angew. Chem. Inter. Ed. Engl., 8, 984 (1969); (b) S. Fliszar,
G. Cardinal, and M. Bernaldin, J. Am. Chem. Soc., 104, 5287 (1982); S. Kuwajima and Z. G. Soos,
J. Am. Chem. Soc., 108, 1707 (1986).
140 G. A. Olah, J. M. Bollinger, and A. M. White, J. Am. Chem. Soc., 91, 3667 (1969); G. A. Olah and
G. D. Mateescu, J. Am. Chem. Soc., 92, 1430 (1970).
141
J. S. McKennis, L. Brener, J. R. Schweiger, and R. Pettit, J. Chem. Soc., Chem. Commun., 365 (1972).
142 T. J. Katz, J. Am. Chem. Soc., 82, 3784 (1960).
143
P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, and J. R. van Eikema Hommes, J. Am. Chem.
Soc., 118, 6317 (1996).