Page 754 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 754
1.375 1.422 1.379 737
1.436 1.425 1.381 1.406 1.409 1.402 1.401 1.426
1.377 1.379
1.373 1.435 1.411 1.416 SECTION 8.2
1.447 1.392 The Annulenes
1.369 1.389 1.401 1.381 1.384
1.373 1.401
1.435 1.425 1.402 1.384 1.426
1.417
(C ) (C ) (C )
2
2
1
1.466 1.365 1.455
1.362 1.466 1.359 1.360
1.442 1.442
1.454 1.355 1.455 1.361
1.352
1.455 1.360 1.440 1.361 1.441
1.358
1.470 1.358 1.454 1.368
1.481
1.355 1.475 1.360 1.36 1.439
1.348 1.439 1.361
1.369 1.439 1.454
(C 1 ) (S ) (C )
1
4
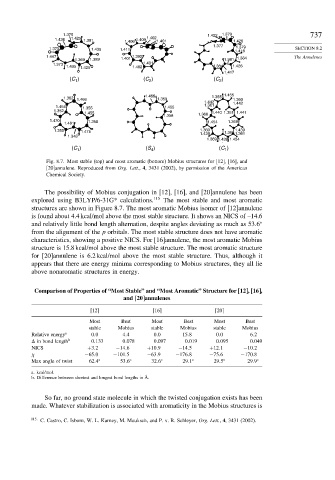
Fig. 8.7. Most stable (top) and most aromatic (bottom) Mobius structures for [12], [16], and
[20]annulene. Reproduced from Org. Lett., 4, 3431 (2002), by permission of the American
Chemical Society.
The possibility of Mobius conjugation in [12], [16], and [20]annulene has been
explored using B3LYP/6-31G* calculations. 115 The most stable and most aromatic
structures are shown in Figure 8.7. The most aromatic Mobius isomer of [12]annulene
is found about 4.4 kcal/mol above the most stable structure. It shows an NICS of –14.6
and relatively little bond length alternation, despite angles deviating as much as 53 6
from the alignment of the p orbitals. The most stable structure does not have aromatic
characteristics, showing a positive NICS. For [16]annulene, the most aromatic Mobius
structure is 15.8 kcal/mol above the most stable structure. The most aromatic structure
for [20]annulene is 6.2 kcal/mol above the most stable structure. Thus, although it
appears that there are energy minima corresponding to Mobius structures, they all lie
above nonaromatic structures in energy.
Comparison of Properties of “Most Stable” and “Most Aromatic” Structure for [12], [16],
and [20]annulenes
[12] [16] [20]
Most Best Most Best Most Best
stable Mobius stable Mobius stable Mobius
Relative energy a 0 0 4 4 0 0 15 8 0 0 6 2
in bond length b 0 133 0 078 0 097 0 019 0 095 0 049
NICS +3 2 −14 6 +10 9 −14 5 +12 1 −10 2
−65 0 −101 5 −63 9 −176 8 −75 6 −170 8
Max angle of twist 62 4 53 6 32 6 29 1 29 5 29 9
a. kcal/mol.
b. Difference between shortest and longest bond lengths in Å.
So far, no ground state molecule in which the twisted conjugation exists has been
made. Whatever stabilization is associated with aromaticity in the Mobius structures is
115
C. Castro, C. Isborn, W. L. Karney, M. Mauksch, and P. v. R. Schleyer, Org. Lett., 4, 3431 (2002).