Page 750 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 750
733
1.428
1.435
1.344 1.342
1.472
1.413 SECTION 8.2
1.360 1.376 1.348
1.448 1.338 1.503
1.383 1.485 The Annulenes
1.378
1.494 1.496
1.453 1.492 1.496 1.437
1.408 1.502 1.500 1.400 1.366 1.352
1.271 1.455
1.450 1.457
1.378 1.374
1.443 1.447 1.390 1.333
1.358
4 9
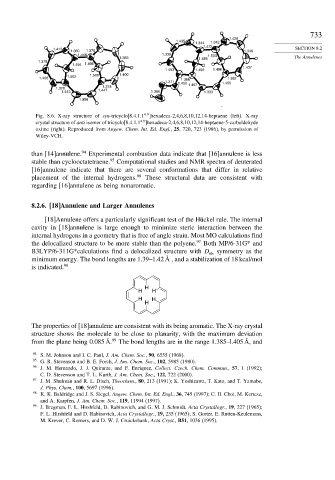
Fig. 8.6. X-ray structure of syn-tricyclo[8.4.1.1 ]hexadeca-2,4,6,8,10,12,14-heptaene (left). X-ray
4 9
crystal structure of anti isomer of tricyclo[8.4.1.1 ]hexadeca-2,4,6,8,10,12,14-heptaene-5-carbaldehyde
oxime (right). Reproduced from Angew. Chem. Int. Ed. Engl., 25, 720, 723 (1986), by permission of
Wiley-VCH.
than [14]annulene. 94 Experimental combustion data indicate that [16]annulene is less
95
stable than cyclooctatetraene. Computational studies and NMR spectra of deuterated
[16]annulene indicate that there are several conformations that differ in relative
placement of the internal hydrogens. 96 These structural data are consistent with
regarding [16]annulene as being nonaromatic.
8.2.6. [18]Annulene and Larger Annulenes
[18]Annulene offers a particularly significant test of the Hückel rule. The internal
cavity in [18]annulene is large enough to minimize steric interaction between the
internal hydrogens in a geometry that is free of angle strain. Most MO calculations find
the delocalized structure to be more stable than the polyene. 97 Both MP/6-31G* and
B3LYP/6-311G*calculations find a delocalized structure with D 6h symmetry as the
minimum energy. The bond lengths are 1.39–1.42 Å , and a stabilization of 18 kcal/mol
is indicated. 98
H
H H
H H
H
The properties of [18]annulene are consistent with its being aromatic. The X-ray crystal
structure shows the molecule to be close to planarity, with the maximum deviation
from the plane being 0.085 Å. 99 The bond lengths are in the range 1.385–l.405 Å, and
94
S. M. Johnson and I. C. Paul, J. Am. Chem. Soc., 90, 6555 (1968).
95 G. R. Stevenson and B. E. Forch, J. Am. Chem. Soc., 102, 5985 (1980).
96 J. M. Hernando, J. J. Quirante, and F. Enriquez, Collect. Czech. Chem. Commun., 57, 1 (1992);
C. D. Stevenson and T. L. Kurth, J. Am. Chem. Soc., 122, 722 (2000).
97
J. M. Shulman and R. L. Disch, Theochem., 80, 213 (1991); K. Yoshizawa, T. Kato, and T. Yamabe,
J. Phys. Chem., 100, 5697 (1996).
98 K. K. Baldridge and J. S. Siegel, Angew. Chem. Int. Ed. Engl., 36, 745 (1997); C. H. Choi, M. Kertesz,
and A. Karpfen, J. Am. Chem. Soc., 119, 11994 (1997).
99
J. Bregman, F. L. Hirshfeld, D. Rabinovich, and G. M. J. Schmidt, Acta Crystallogr., 19, 227 (1965);
F. L. Hirshfeld and D. Rabinovich, Acta Crystallogr., 19, 235 (1965); S. Gorter, E. Rutten-Keulemans,
M. Krever, C. Romers, and D. W. J. Cruickshank, Acta Cryst., B51, 1036 (1995).