Page 747 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 747
730
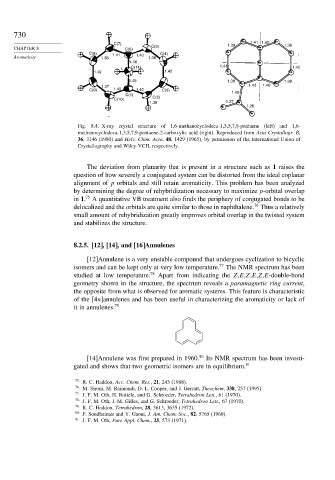
C(7) 1.39 1.41 1.40 1.38
CHAPTER 8 C(6) C(5)
C(8) 1.41 1.40 C(4)
Aromaticity 1.38 1.38
1.48
C(11) 1.41 1.42
1.42 1.42
1.49 1.38 1.39
1.43 1.40
1.37
C(9) 1.40 1.40 C(3) 1.49
C(1) C(2)
C(10)
1.38 1.27
1.26
Fig. 8.4. X-ray crystal structure of 1,6-methanocyclodeca-1,3,5,7,9-pentaene (left) and 1,6-
methanocyclodeca-1,3,5,7,9-pentaene-2-carboxylic acid (right). Reproduced from Acta Crystallogr. B,
36, 3146 (1980) and Helv. Chim. Acta, 48, 1429 (1965), by permission of the International Union of
Crystallogrophy and Wiley-VCH, respectively.
The deviation from planarity that is present in a structure such as 1 raises the
question of how severely a conjugated system can be distorted from the ideal coplanar
alignment of p orbitals and still retain aromaticity. This problem has been analyzed
by determining the degree of rehybridization necessary to maximize p-orbital overlap
75
in 1. A quantitative VB treatment also finds the periphery of conjugated bonds to be
76
delocalized and the orbitals are quite similar to those in naphthalene. Thus a relatively
small amount of rehybridization greatly improves orbital overlap in the twisted system
and stabilizes the structure.
8.2.5. [12], [14], and [16]Annulenes
[12]Annulene is a very unstable compound that undergoes cyclization to bicyclic
77
isomers and can be kept only at very low temperature. The NMR spectrum has been
studied at low temperature. 78 Apart from indicating the Z,E,Z,E,Z,E-double-bond
geometry shown in the structure, the spectrum reveals a paramagnetic ring current,
the opposite from what is observed for aromatic systems. This feature is characteristic
of the [4n]annulenes and has been useful in characterizing the aromaticity or lack of
it in annulenes. 79
[14]Annulene was first prepared in 1960. 80 Its NMR spectrum has been investi-
gated and shows that two geometric isomers are in equilibrium. 81
75 R. C. Haddon, Acc. Chem. Res., 21, 243 (1988).
76
M. Sironi, M. Raimondi, D. L. Cooper, and J. Gerratt, Theochem, 338, 257 (1995).
77 J. F. M. Oth, H. Rottele, and G. Schroeder, Tetrahedron Lett., 61 (1970).
78
J. F. M. Oth, J.-M. Gilles, and G. Schroeder, Tetrahedron Lett., 67 (1970).
79
R. C. Haddon, Tetrahedron, 28, 3613, 3635 (1972).
80 F. Sondheimer and Y. Gaoni, J. Am. Chem. Soc., 82, 5765 (1960).
81
J. F. M. Oth, Pure Appl. Chem., 25, 573 (1971).