Page 745 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 745
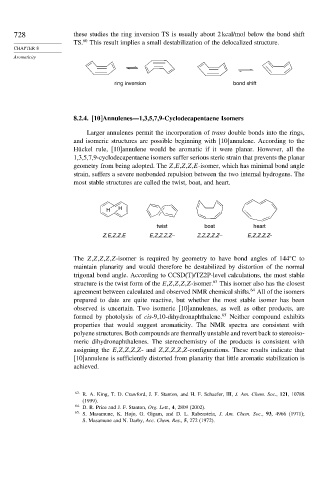
728 these studies the ring inversion TS is usually about 2 kcal/mol below the bond shift
TS. 60 This result implies a small destabilization of the delocalized structure.
CHAPTER 8
Aromaticity
ring inversion bond shift
8.2.4. [10]Annulenes—1,3,5,7,9-Cyclodecapentaene Isomers
Larger annulenes permit the incorporation of trans double bonds into the rings,
and isomeric structures are possible beginning with [10]annulene. According to the
Hückel rule, [10]annulene would be aromatic if it were planar. However, all the
1,3,5,7,9-cyclodecapentaene isomers suffer serious steric strain that prevents the planar
geometry from being adopted. The Z,E,Z,Z,E-isomer, which has minimal bond angle
strain, suffers a severe nonbonded repulsion between the two internal hydrogens. The
most stable structures are called the twist, boat, and heart.
H H
twist boat heart
Z,E,Z,Z,E E,Z,Z,Z,Z– Z,Z,Z,Z,Z– E,Z,Z,Z,Z-
The Z,Z,Z,Z,Z-isomer is required by geometry to have bond angles of 144 Cto
maintain planarity and would therefore be destabilized by distortion of the normal
trigonal bond angle. According to CCSD(T)/TZ2P-level calculations, the most stable
63
structure is the twist form of the E,Z,Z,Z,Z-isomer. This isomer also has the closest
64
agreement between calculated and observed NMR chemical shifts. All of the isomers
prepared to date are quite reactive, but whether the most stable isomer has been
observed is uncertain. Two isomeric [10]annulenes, as well as other products, are
formed by photolysis of cis-9,10-dihydronaphthalene. 65 Neither compound exhibits
properties that would suggest aromaticity. The NMR spectra are consistent with
polyene structures. Both compounds are thermally unstable and revert back to stereoiso-
meric dihydronaphthalenes. The stereochemistry of the products is consistent with
assigning the E,Z,Z,Z,Z- and Z,Z,Z,Z,Z-configurations. These results indicate that
[10]annulene is sufficiently distorted from planarity that little aromatic stabilization is
achieved.
63 R. A. King, T. D. Crawford, J. F. Stanton, and H. F. Schaefer, III, J. Am. Chem. Soc., 121, 10788
(1999).
64 D. R. Price and J. F. Stanton, Org. Lett., 4, 2809 (2002).
65
S. Masamune, K. Hojo, G. Gigam, and D. L. Rabenstein, J. Am. Chem. Soc., 93, 4966 (1971);
S. Masamune and N. Darby, Acc. Chem. Res., 5, 272 (1972).