Page 741 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 741
724
CHAPTER 8
Aromaticity
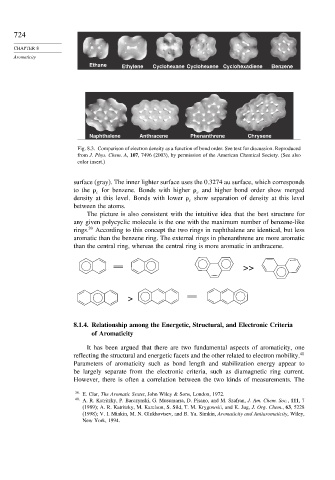
Ethane Ethylene Cyclohexane Cyclohexene Cyclohexadiene Benzene
Naphthalene Anthracene Phenanthrene Chrysene
Fig. 8.3. Comparison of electron density as a function of bond order. See text for discussion. Reproduced
from J. Phys. Chem. A, 107, 7496 (2003), by permission of the American Chemical Society. (See also
color insert.)
surface (gray). The inner lighter surface uses the 0.3274 au surface, which corresponds
to the for benzene. Bonds with higher and higher bond order show merged
c
c
density at this level. Bonds with lower show separation of density at this level
c
between the atoms.
The picture is also consistent with the intuitive idea that the best structure for
any given polycyclic molecule is the one with the maximum number of benzene-like
rings. 39 According to this concept the two rings in naphthalene are identical, but less
aromatic than the benzene ring. The external rings in phenanthrene are more aromatic
than the central ring, whereas the central ring is more aromatic in anthracene.
>>
>
8.1.4. Relationship among the Energetic, Structural, and Electronic Criteria
of Aromaticity
It has been argued that there are two fundamental aspects of aromaticity, one
reflecting the structural and energetic facets and the other related to electron mobility. 40
Parameters of aromaticity such as bond length and stabilization energy appear to
be largely separate from the electronic criteria, such as diamagnetic ring current.
However, there is often a correlation between the two kinds of measurements. The
39 E. Clar, The Aromatic Sextet, John Wiley & Sons, London, 1972.
40
A. R. Katritzky, P. Barczynski, G. Musumarra, D. Pisano, and M. Szafran, J. Am. Chem. Soc., 111,7
(1989); A. R. Katritzky, M. Karelson, S. Sild, T. M. Krygowski, and K. Jug, J. Org. Chem., 63, 5228
(1998); V. I. Minkin, M. N. Glukhovtsev, and B. Ya. Simkin, Aromaticity and Antiaromaticity, Wiley,
New York, 1994.