Page 740 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 740
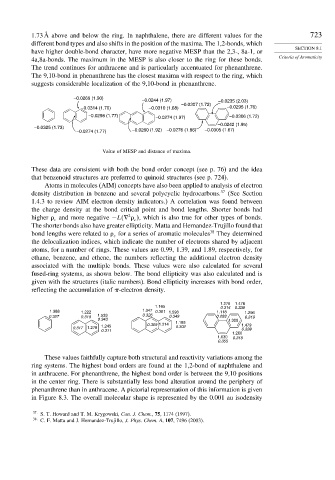
1.73 Å above and below the ring. In naphthalene, there are different values for the 723
different bond types and also shifts in the position of the maxima. The 1,2-bonds, which
SECTION 8.1
have higher double-bond character, have more negative MESP than the 2,3-, 8a-1, or
Criteria of Aromaticity
4a,8a-bonds. The maximum in the MESP is also closer to the ring for these bonds.
The trend continues for anthracene and is particularly accentuated for phenanthrene.
The 9,10-bond in phenanthrene has the closest maxima with respect to the ring, which
suggests considerable localization of the 9,10-bond in phenanthrene.
–0.0269 (1.90)
–0.0244 (1.97) –0.0235 (2.03)
–0.0307 (1.72)
–0.0314 (1.70) –0.0310 (1.68) –0.0295 (1.76)
–0.0298 (1.77) –0.0274 (1.97) –0.0306 (1.72)
–0.0242 (1.95)
–0.0325 (1.73)
–0.0274 (1.77) –0.0260 (1.92) –0.0276 (1.86) –0.0305 (1.67)
Value of MESP and distance of maxima.
These data are consistent with both the bond order concept (see p. 76) and the idea
that benzenoid structures are preferred to quinoid structures (see p. 724).
Atoms in molecules (AIM) concepts have also been applied to analysis of electron
density distribution in benzene and several polycyclic hydrocarbons. 37 (See Section
1.4.3 to review AIM electron density indicators.) A correlation was found between
the charge density at the bond critical point and bond lengths. Shorter bonds had
2
higher and more negative −L , which is also true for other types of bonds.
c c
The shorter bonds also have greater ellipticity. Matta and Hernandez-Trujillo found that
bond lengths were related to for a series of aromatic molecules 38 They determined
c
the delocalization indices, which indicate the number of electrons shared by adjacent
atoms, for a number of rings. These values are 0.99, 1.39, and 1.89, respectively, for
ethane, benzene, and ethene, the numbers reflecting the additional electron density
associated with the multiple bonds. These values were also calculated for several
fused-ring systems, as shown below. The bond ellipticity was also calculated and is
given with the structures (italic numbers). Bond ellipticity increases with bond order,
reflecting the accumulation of -electron density.
1.276 1.476
1.165 0.314 0.338
1.388 1.222 1.347 0.301 1.598 1.118 1.296
0.327 0.310 1.533 0.326 0.349 0.288 0.319
0.343 1.185 1.308
0.317 1.278 1.245 0.309 1.214 0.302 1.479
0.339
0.311
1.260
1.630 0.316
0.355
These values faithfully capture both structural and reactivity variations among the
ring systems. The highest bond orders are found at the 1,2-bond of naphthalene and
in anthracene. For phenanthrene, the highest bond order is between the 9,10 positions
in the center ring. There is substantially less bond alteration around the periphery of
phenanthrene than in anthracene. A pictorial representation of this information is given
in Figure 8.3. The overall molecular shape is represented by the 0.001 au isodensity
37 S. T. Howard and T. M. Krygowski, Can. J. Chem., 75, 1174 (1997).
38
C. F. Matta and J. Hernandez-Trujillo, J. Phys. Chem. A, 107, 7496 (2003).