Page 739 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 739
722 theoretically and defined in such a way that it can be used to compare different rings.
The units are nanoamperes (nA), and the calculated ring currents are found to increase
CHAPTER 8 in more electron-rich systems, as is the case with NICS. Whereas benzene is 32 nA,
Aromaticity pyrrole is 41.4 nA and cyclopentadienide is 72.2 nA. Conversely, it is lower for the
cycloheptatrienyl cation (26.1 nA); thiophene (32.1 nA) is very similar to benzene;
and nonaromatic compounds such as cyclohexane or 1,4-cyclohexadiene have much
smaller values (2.1 and 1.7 nA, respectively).
Another electronic property associated with aromaticity is magnetic susceptibility,
which is determined by measuring the force exerted on the sample by a magnetic
field. 31 Magnetic susceptibility is closely related to polarizability and is different
in the plane and perpendicular to the plane of the ring. It can be determined by
various spectroscopic measurements, 32 as well as by using an NMR spectrometer. 33
It is observed that aromatic compounds have enhanced magnetic susceptibility, called
exaltation ( , relative to values predicted on the basis of the localized structural
components. 34
Magnetic Susceptibility Exaltation for Some
Aromatic Hydrocarbons
Compound
Benzene 13 7
Naphthalene 30 5
Anthracene 48 6
Phenanthrene 46 2
Azulene 29 6
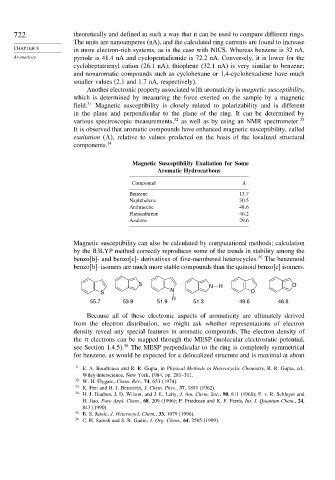
Magnetic susceptibility can also be calculated by computational methods; calculation
by the B3LYP method correctly reproduces some of the trends in stability among the
benzo[b]- and benzo[c]- derivatives of five-membered heterocycles. 35 The benzenoid
benzo[b]- isomers are much more stable compounds than the quinoid benzo[c] isomers.
S N H O
N
S O
H
55.7 53.9 51.9 51.3 49.6 46.8
Because all of these electronic aspects of aromaticity are ultimately derived
from the electron distribution, we might ask whether representations of electron
density reveal any special features in aromatic compounds. The electron density of
the electrons can be mapped through the MESP (molecular electrostatic potential,
see Section 1.4.5). 36 The MESP perpendicular to the ring is completely symmetrical
for benzene, as would be expected for a delocalized structure and is maximal at about
31
E. A. Boudreaux and R. R. Gupta, in Physical Methods in Heterocyclic Chemistry, R. R. Gupta, ed.,
Wiley-Interscience, New York, 1984, pp. 281–311.
32 W. H. Flygare, Chem. Rev., 74, 653 (1974).
33
K. Frei and H. J. Bernstein, J. Chem. Phys., 37, 1891 (1962).
34 H. J. Dauben, J. D. Wilson, and J. L. Laity, J. Am. Chem. Soc., 90, 811 (1968); P. v. R. Schleyer and
H. Jiao, Pure Appl. Chem., 68, 209 (1996); P. Friedman and K. F. Ferris, Int. J. Quantum Chem., 24,
843 (1990).
35 B. S. Jursic, J. Heterocycl. Chem., 33, 1079 (1996).
36
C. H. Suresh and S. R. Gadre, J. Org. Chem., 64, 2505 (1999).