Page 734 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 734
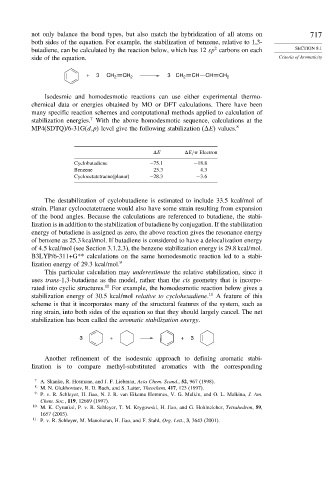
not only balance the bond types, but also match the hybridization of all atoms on 717
both sides of the equation. For example, the stabilization of benzene, relative to 1,3-
2
butadiene, can be calculated by the reaction below, which has 12 sp carbons on each SECTION 8.1
side of the equation. Criteria of Aromaticity
+ 3 CH 2 CH 2 3 CH 2 CH CH CH 2
Isodesmic and homodesmotic reactions can use either experimental thermo-
chemical data or energies obtained by MO or DFT calculations. There have been
many specific reaction schemes and computational methods applied to calculation of
7
stabilization energies. With the above homodesmotic sequence, calculations at the
MP4(SDTQ)/6-31G(d,p level give the following stabilization ( E values. 8
E E/ Electron
Cyclobutadiene −75 1 −18 8
Benzene 25 3 4 3
Cyclooctatetraene(planar) −28 3 −3 6
The destabilization of cyclobutadiene is estimated to include 33.5 kcal/mol of
strain. Planar cyclooctatetraene would also have some strain resulting from expansion
of the bond angles. Because the calculations are referenced to butadiene, the stabi-
lization is in addition to the stabilization of butadiene by conjugation. If the stabilization
energy of butadiene is assigned as zero, the above reaction gives the resonance energy
of benzene as 25.3 kcal/mol. If butadiene is considered to have a delocalization energy
of 4.5 kcal/mol (see Section 3.1.2.3), the benzene stabilization energy is 29.8 kcal/mol.
B3LYP/6-311+G** calculations on the same homodesmotic reaction led to a stabi-
lization energy of 29.3 kcal/mol. 9
This particular calculation may underestimate the relative stabilization, since it
uses trans-1,3-butadiene as the model, rather than the cis geometry that is incorpo-
rated into cyclic structures. 10 For example, the homodesmotic reaction below gives a
stabilization energy of 30.5 kcal/mol relative to cyclohexadiene. 11 A feature of this
scheme is that it incorporates many of the structural features of the system, such as
ring strain, into both sides of the equation so that they should largely cancel. The net
stabilization has been called the aromatic stabilization energy.
3 + + 3
Another refinement of the isodesmic approach to defining aromatic stabi-
lization is to compare methyl-substituted aromatics with the corresponding
7 A. Skanke, R. Hosmane, and J. F. Liebman, Acta Chem. Scand., 52, 967 (1998).
8 M. N. Glukhovtsev, R. D. Bach, and S. Laiter, Theochem, 417, 123 (1997).
9
P. v. R. Schleyer, H. Jiao, N. J. R. van Eikema Hommes, V. G. Malkin, and O. L. Malkina, J. Am.
Chem. Soc., 119, 12669 (1997).
10 M. K. Cyranksi, P. v. R. Schleyer, T. M. Krygowski, H. Jiao, and G. Hohlneicher, Tetrahedron, 59,
1657 (2003).
11
P. v. R. Schleyer, M. Manoharan, H. Jiao, and F. Stahl, Org. Lett., 3, 3643 (2001).