Page 735 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 735
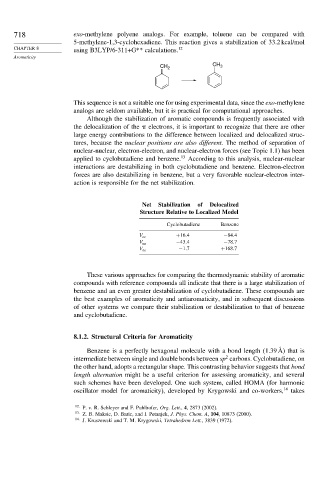
718 exo-methylene polyene analogs. For example, toluene can be compared with
5-methylene-1,3-cyclohexadiene. This reaction gives a stabilization of 33.2 kcal/mol
CHAPTER 8 using B3LYP/6-311+G** calculations. 12
Aromaticity
CH 2 CH 3
This sequence is not a suitable one for using experimental data, since the exo-methylene
analogs are seldom available, but it is practical for computational approaches.
Although the stabilization of aromatic compounds is frequently associated with
the delocalization of the electrons, it is important to recognize that there are other
large energy contributions to the difference between localized and delocalized struc-
tures, because the nuclear positions are also different. The method of separation of
nuclear-nuclear, electron-electron, and nuclear-electron forces (see Topic 1.1) has been
applied to cyclobutadiene and benzene. 13 According to this analysis, nuclear-nuclear
interactions are destabilizing in both cyclobutadiene and benzene. Electron-electron
forces are also destabilizing in benzene, but a very favorable nuclear-electron inter-
action is responsible for the net stabilization.
Net Stabilization of Delocalized
Structure Relative to Localized Model
Cyclobutadiene Benzene
+16 4 −84 4
V ee
−43 4 −78 7
V nn
−1 7 +168 7
V ne
These various approaches for comparing the thermodynamic stability of aromatic
compounds with reference compounds all indicate that there is a large stabilization of
benzene and an even greater destabilization of cyclobutadiene. These compounds are
the best examples of aromaticity and antiaromaticity, and in subsequent discussions
of other systems we compare their stabilization or destabilization to that of benzene
and cyclobutadiene.
8.1.2. Structural Criteria for Aromaticity
Benzene is a perfectly hexagonal molecule with a bond length (1.39 Å) that is
2
intermediate between single and double bonds between sp carbons. Cyclobutadiene, on
the other hand, adopts a rectangular shape. This contrasting behavior suggests that bond
length alternation might be a useful criterion for assessing aromaticity, and several
such schemes have been developed. One such system, called HOMA (for harmonic
oscillator model for aromaticity), developed by Krygowski and co-workers, 14 takes
12
P. v. R. Schleyer and F. Puhlhofer, Org. Lett., 4, 2873 (2002).
13 Z. B. Maksic, D. Baric, and I. Petanjek, J. Phys. Chem. A, 104, 10873 (2000).
14
J. Kruszewski and T. M. Krygowski, Tetrahedron Lett., 3839 (1972).