Page 746 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 746
H 729
hv
+ SECTION 8.2
The Annulenes
H
H H
H H
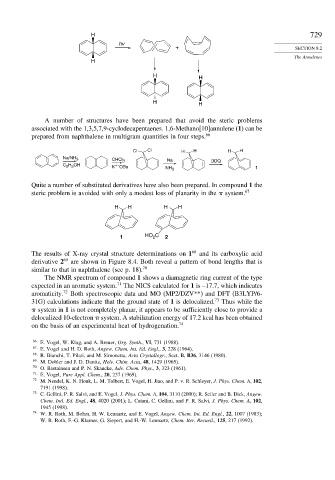
A number of structures have been prepared that avoid the steric problems
associated with the 1,3,5,7,9-cyclodecapentaenes. 1,6-Methano[10]annulene (1) can be
prepared from naphthalene in multigram quantities in four steps. 66
Cl Cl H H H H
Na/NH 3
CHCl 3 Na DDQ
C 2 H 5 OH K +– OBu
NH 3 1
Quite a number of substituted derivatives have also been prepared. In compound 1 the
steric problem is avoided with only a modest loss of planarity in the system. 67
H H H H
1 HO C 2
2
The results of X-ray crystal structure determinations on 1 68 and its carboxylic acid
derivative 2 69 are shown in Figure 8.4. Both reveal a pattern of bond lengths that is
similar to that in naphthalene (see p. 18). 70
The NMR spectrum of compound 1 shows a diamagnetic ring current of the type
71
expected in an aromatic system. The NICS calculated for 1 is –17.7, which indicates
aromaticity. 72 Both spectroscopic data and MO (MP2/DZV**) and DFT (B3LYP/6-
31G) calculations indicate that the ground state of 1 is delocalized. 73 Thus while the
system in 1 is not completely planar, it appears to be sufficiently close to provide a
delocalized 10-electron system. A stabilization energy of 17.2 kcal has been obtained
on the basis of an experimental heat of hydrogenation. 74
66
E. Vogel, W. Klug, and A. Breuer, Org. Synth., VI, 731 (1988).
67 E. Vogel and H. D. Roth, Angew. Chem. Int. Ed. Engl., 3, 228 (1964).
68 R. Bianchi, T. Pilati, and M. Simonetta, Acta Crystallogr., Sect. B, B36, 3146 (1980).
69
M. Dobler and J. D. Dunitz, Helv. Chim. Acta, 48, 1429 (1965).
70
O. Bastainsen and P. N. Skancke, Adv. Chem. Phys., 3, 323 (1961).
71 E. Vogel, Pure Appl. Chem., 20, 237 (1969).
72
M. Nendel, K. N. Houk, L. M. Tolbert, E. Vogel, H. Jiao, and P. v. R. Schleyer, J. Phys. Chem. A, 102,
7191 (1998).
73 C. Gellini, P. R. Salvi, and E. Vogel, J. Phys. Chem. A, 104, 3110 (2000); R. Seiler and B. Dick, Angew.
Chem. Intl. Ed. Engl., 48, 4020 (2001); L. Catani, C. Gellini, and P. R. Salvi, J. Phys. Chem. A, 102,
1945 (1998).
74
W. R. Roth, M. Bohm, H. W. Lennartz, and E. Vogel, Angew. Chem. Int. Ed. Engl., 22, 1007 (1983);
W. R. Roth, F.-G. Klarner, G. Siepert, and H.-W. Lennartz, Chem. Ber. Recueil., 125, 217 (1992).