Page 749 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 749
o
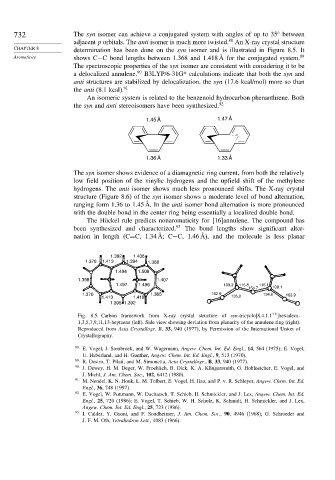
732 The syn isomer can achieve a conjugated system with angles of up to 35 between
88
adjacent p orbitals. The anti isomer is much more twisted. An X-ray crystal structure
CHAPTER 8 determination has been done on the syn isomer and is illustrated in Figure 8.5. It
Aromaticity shows C−C bond lengths between 1.368 and 1.418 Å for the conjugated system. 89
The spectroscopic properties of the syn isomer are consistent with considering it to be
a delocalized annulene. 90 B3LYP/6-31G* calculations indicate that both the syn and
anti structures are stabilized by delocalization, the syn (17.6 kcal/mol) more so than
the anti (8.1 kcal). 91
An isomeric system is related to the benzenoid hydrocarbon phenanthrene. Both
the syn and anti stereoisomers have been synthesized. 92
1.45 Å 1.47 Å
1.36 Å 1.33 Å
The syn isomer shows evidence of a diamagnetic ring current, from both the relatively
low field position of the vinylic hydrogens and the upfield shift of the methylene
hydrogens. The anti isomer shows much less pronounced shifts. The X-ray crystal
structure (Figure 8.6) of the syn isomer shows a moderate level of bond alternation,
ranging form 1.36 to 1.45 Å. In the anti isomer bond alternation is more pronounced
with the double bond in the center ring being essentially a localized double bond.
The Hückel rule predicts nonaromaticity for [16]annulene. The compound has
been synthesized and characterized. 93 The bond lengths show significant alter-
nation in length (C=C, 1.34 Å; C−C, 1.46 Å), and the molecule is less planar
1.392 1.406
1.376 1.413 1.394 1.368
1.494 1.508
1.398 1.407
1.497 1.496 109.2 115.8 116.1
154.7 109.1
1.370 1.365 162.9 134.6 163.9
1.413 1.418 135.0
1.395 1.392
3 8
Fig. 8.5. Carbon framework from X-ray crystal structure of syn-tricyclo[8.4.1.1 ]hexadeca-
1,3,5,7,9,11,13-heptaene (left). Side view showing deviation from planarity of the annulene ring (right).
Reproduced from Acta Crystallogr. B, 33, 940 (1977), by Permission of the International Union of
Crystallography.
88 E. Vogel, J. Sombroek, and W. Wagemann, Angew. Chem. Int. Ed. Engl., 14, 564 (1975); E. Vogel,
U. Haberland, and H. Gunther, Angew. Chem. Int. Ed. Engl., 9, 513 (1970).
89
R. Destro, T. Pilati, and M. Simonetta, Acta Crystallogr., B, 33, 940 (1977).
90 J. Dewey, H. M. Deger, W. Froehlich, B. Dick, K. A. Klingensmith, G. Hohlneicher, E. Vogel, and
J. Michl, J. Am. Chem. Soc., 102, 6412 (1980).
91
M. Nendel, K. N. Houk, L. M. Tolbert, E. Vogel, H. Jiao, and P. v. R. Schleyer, Angew. Chem. Int. Ed.
Engl., 36, 748 (1997).
92 E. Vogel, W. Puttmann, W. Duchatsch, T. Schieb, H. Schmickler, and J. Lex, Angew. Chem. Int. Ed.
Engl., 25, 720 (1986); E. Vogel, T. Schieb, W. H. Schulz, K. Schmidt, H. Schmickler, and J. Lex,
Angew. Chem. Int. Ed. Engl., 25, 723 (1986).
93
I. Calder, Y. Gaoni, and F. Sondheimer, J. Am. Chem. Soc., 90, 4946 (1968); G. Schroeder and
J. F. M. Oth, Tetrahedron Lett., 4083 (1966).