Page 755 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 755
738 counterbalanced by bond angle and other strain, so the prediction of Mobius aromaticity
remains to be verified experimentally. Its correctness is strongly suggested, however,
CHAPTER 8 by the fact that transition structures with twisted orbital arrays appear to be perfectly
Aromaticity acceptable in many organic reactions. 116 We return to this topic in Section 10.1. The
rules for aromaticity can be generalized to include Mobius orbital arrays:
Hückel Orbital Array Mobius Orbital Array
4n+2 = Aromatic 4n = Aromatic
4n = Antiaromatic 4n+2 = Antiaromatic
8.3. Aromaticity in Charged Rings
There are striking stability relationships owing to aromaticity in charged ring
systems. The HMO energy levels that apply to fully conjugated planar three- to
nine-membered rings were shown earlier in Figure 8.1 (p. 714). These energy levels
are applicable to ions as well as to the neutral annulenes. A number of cations and
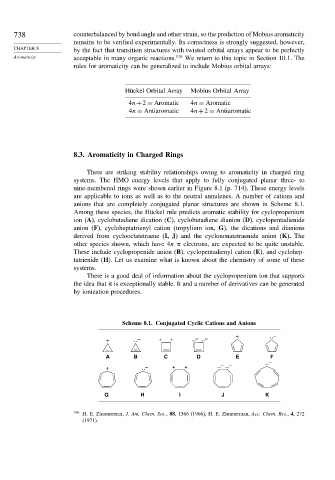
anions that are completely conjugated planar structures are shown in Scheme 8.1.
Among these species, the Hückel rule predicts aromatic stability for cyclopropenium
ion (A), cyclobutadiene dication (C), cyclobutadiene dianion (D), cyclopentadienide
anion (F), cycloheptatrienyl cation (tropylium ion, G), the dications and dianions
derived from cyclooctatetraene (I, J) and the cyclononatetraenide anion (K). The
other species shown, which have 4n electrons, are expected to be quite unstable.
These include cyclopropenide anion (B), cyclopentadienyl cation (E), and cyclohep-
tatrienide (H). Let us examine what is known about the chemistry of some of these
systems.
There is a good deal of information about the cyclopropenium ion that supports
the idea that it is exceptionally stable. It and a number of derivatives can be generated
by ionization procedures.
Scheme 8.1. Conjugated Cyclic Cations and Anions
+ –
+ – + + – –
A B C D E F
–
– –
+ – + +
G H I J K
116
H. E. Zimmerman, J. Am. Chem. Soc., 88, 1566 (1966); H. E. Zimmerman, Acc. Chem. Res., 4, 272
(1971).