Page 760 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 760
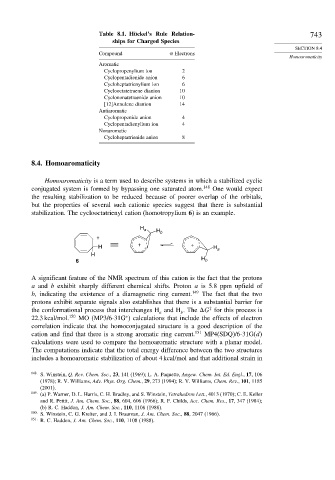
Table 8.1. Hückel’s Rule Relation- 743
ships for Charged Species
SECTION 8.4
Compound Electrons
Homoaromaticity
Aromatic
Cyclopropenylium ion 2
Cyclopentadienide anion 6
Cycloheptatrienylium ion 6
Cyclooctatetraene dianion 10
Cyclononatetraenide anion 10
[12]Annulene dianion 14
Antiaromatic
Cyclopropenide anion 4
Cyclopentadienylium ion 4
Nonaromatic
Cycloheptatrienide anion 8
8.4. Homoaromaticity
Homoaromaticity is a term used to describe systems in which a stabilized cyclic
conjugated system is formed by bypassing one saturated atom. 148 One would expect
the resulting stabilization to be reduced because of poorer overlap of the orbitals,
but the properties of several such cationic species suggest that there is substantial
stabilization. The cyclooctatrienyl cation (homotropylium 6) is an example.
H a H b
+
+ +
H H a
H
H
6 b
A significant feature of the NMR spectrum of this cation is the fact that the protons
a and b exhibit sharply different chemical shifts. Proton a is 5.8 ppm upfield of
b, indicating the existence of a diamagnetic ring current. 149 The fact that the two
protons exhibit separate signals also establishes that there is a substantial barrier for
‡
the conformational process that interchanges H and H . The G for this process is
b
a
22.3 kcal/mol. 150 MO (MP3/6-31G ) calculations that include the effects of electron
∗
correlation indicate that the homoconjugated structure is a good description of the
cation and find that there is a strong aromatic ring current. 151 MP4(SDQ)/6-31G(d
calculations were used to compare the homoaromatic structure with a planar model.
The computations indicate that the total energy difference between the two structures
includes a homoaromatic stabilization of about 4 kcal/mol and that additional strain in
148
S. Winstein, Q. Rev. Chem. Soc., 23, 141 (1969); L. A. Paquette, Angew. Chem. Int. Ed. Engl., 17, 106
(1978); R. V. Williams, Adv. Phys. Org. Chem., 29, 273 (1994); R. V. Williams, Chem. Rev., 101, 1185
(2001).
149 (a) P. Warner, D. L. Harris, C. H. Bradley, and S. Winstein, Tetrahedron Lett., 4013 (1970); C. E. Keller
and R. Pettit, J. Am. Chem. Soc., 88, 604, 606 (1966); R. F. Childs, Acc. Chem. Res., 17, 347 (1984);
(b) R. C. Haddon, J. Am. Chem. Soc., 110, 1108 (1988).
150 S. Winstein, C. G. Kreiter, and J. I. Brauman, J. Am. Chem. Soc., 88, 2047 (1966).
151
R. C. Haddon, J. Am. Chem. Soc., 110, 1108 (1988).