Page 825 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 825
808 decreases as the size of the entering alkyl group increases along the series methyl,
ethyl, i-propyl. 89 No ortho product is found when the entering group is t-butyl. 90
CHAPTER 9
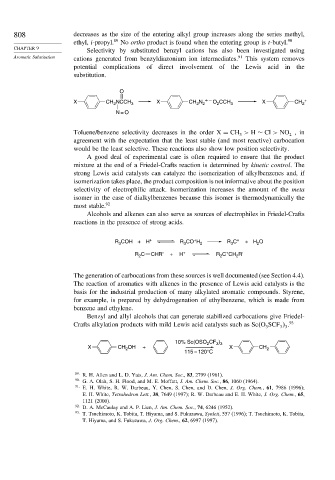
Selectivity by substituted benzyl cations has also been investigated using
Aromatic Substitution cations generated from benzyldiazonium ion intermediates. 91 This system removes
potential complications of direct involvement of the Lewis acid in the
substitution.
O
+ – +
X CH NCCH 3 X CH N O CCH 3 X CH 2
2
2
2 2
N = O
Toluene/benzene selectivity decreases in the order X = CH > H ∼ Cl > NO ,in
3
2
agreement with the expectation that the least stable (and most reactive) carbocation
would be the least selective. These reactions also show low position selectivity.
A good deal of experimental care is often required to ensure that the product
mixture at the end of a Friedel-Crafts reaction is determined by kinetic control. The
strong Lewis acid catalysts can catalyze the isomerization of alkylbenzenes and, if
isomerization takes place, the product composition is not informative about the position
selectivity of electrophilic attack. Isomerization increases the amount of the meta
isomer in the case of dialkylbenzenes because this isomer is thermodynamically the
most stable. 92
Alcohols and alkenes can also serve as sources of electrophiles in Friedel-Crafts
reactions in the presence of strong acids.
+
R COH + H + R CO H 2 R C + + H O
3
3
3
2
+
R C CHR' + H + R C CH R'
2
2
2
The generation of carbocations from these sources is well documented (see Section 4.4).
The reaction of aromatics with alkenes in the presence of Lewis acid catalysts is the
basis for the industrial production of many alkylated aromatic compounds. Styrene,
for example, is prepared by dehydrogenation of ethylbenzene, which is made from
benzene and ethylene.
Benzyl and allyl alcohols that can generate stabilized carbocations give Friedel-
Crafts alkylation products with mild Lewis acid catalysts such as Sc O SCF . 93
3
3 3
10% Sc(OSO CF )
2
3 3
X CH OH + X CH 2
2
115 – 120°C
89 R. H. Allen and L. D. Yats, J. Am. Chem. Soc., 83, 2799 (1961).
90 G. A. Olah, S. H. Flood, and M. E. Moffatt, J. Am. Chem. Soc., 86, 1060 (1964).
91
E. H. White, R. W. Darbeau, Y. Chen, S. Chen, and D. Chen, J. Org. Chem., 61, 7986 (1996);
E. H. White, Tetrahedron Lett., 38, 7649 (1997); R. W. Darbeau and E. H. White, J. Org. Chem., 65,
1121 (2000).
92 D. A. McCaulay and A. P. Lien, J. Am. Chem. Soc., 74, 6246 (1952).
93
T. Tsuchimoto, K. Tobita, T. Hiyama, and S. Fukuzawa, Synlett, 557 (1996); T. Tsuchimoto, K. Tobita,
T. Hiyama, and S. Fukuzawa, J. Org. Chem., 62, 6997 (1997).