Page 830 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 830
–
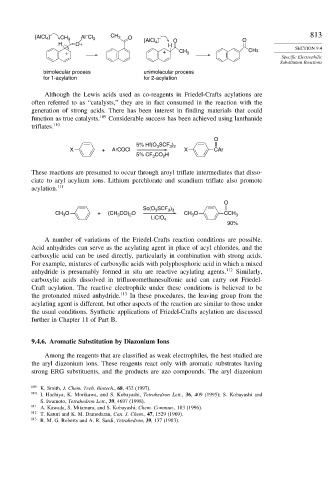
[AlCl 4 ] – CH 3 Al Cl 3 CH 3 O [AlCl 4 ] – O 813
H O+ H O SECTION 9.4
+ + CH 3 CH3
Specific Electrophilic
Substitution Reactions
bimolecular process unimolecular process
for 1-acylation for 2-acylation
Although the Lewis acids used as co-reagents in Friedel-Crafts acylations are
often referred to as “catalysts,” they are in fact consumed in the reaction with the
generation of strong acids. There has been interest in finding materials that could
function as true catalysts. 109 Considerable success has been achieved using lanthanide
triflates. 110
O
5% Hf(O SCF )
3
3 2
X + ArCOCl X CAr
5% CF CO H
3
2
These reactions are presumed to occur through aroyl triflate intermediates that disso-
ciate to aryl acylium ions. Lithium perchlorate and scandium triflate also promote
acylation. 111
O
Sc(O SCF )
3 3
3
CH O + (CH CO) O CH O CCH 3
3
2
3
3
LiClO 4
90%
A number of variations of the Friedel-Crafts reaction conditions are possible.
Acid anhydrides can serve as the acylating agent in place of acyl chlorides, and the
carboxylic acid can be used directly, particularly in combination with strong acids.
For example, mixtures of carboxylic acids with polyphosphoric acid in which a mixed
anhydride is presumably formed in situ are reactive acylating agents. 112 Similarly,
carboxylic acids dissolved in trifluoromethanesulfonic acid can carry out Friedel-
Craft acylation. The reactive electrophile under these conditions is believed to be
the protonated mixed anhydride. 113 In these procedures, the leaving group from the
acylating agent is different, but other aspects of the reaction are similar to those under
the usual conditions. Synthetic applications of Friedel-Crafts acylation are discussed
further in Chapter 11 of Part B.
9.4.6. Aromatic Substitution by Diazonium Ions
Among the reagents that are classified as weak electrophiles, the best studied are
the aryl diazonium ions. These reagents react only with aromatic substrates having
strong ERG substituents, and the products are azo compounds. The aryl diazonium
109 K. Smith, J. Chem. Tech. Biotech., 68, 432 (1997).
110 I. Hachiya, K. Morikawa, and S. Kobayashi, Tetrahedron Lett., 36, 409 (1995); S. Kobayashi and
S. Iwamoto, Tetrahedron Lett., 39, 4697 (1998).
111
A. Kawada, S. Mitamura, and S. Kobayashi, Chem. Commun., 183 (1996).
112 T. Katuri and K. M. Damodaran, Can. J. Chem., 47, 1529 (1969).
113
R. M. G. Roberts and A. R. Sardi, Tetrahedron, 39, 137 (1983).