Page 831 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 831
814 ions are usually generated by diazotization of aromatic amines. The mechanism of
diazonium ion formation is discussed more completely in Section 11.2.1 of Part B.
CHAPTER 9
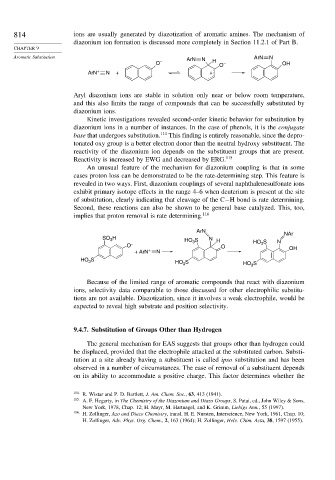
Aromatic Substitution ArN N ArN N
O – H O – OH
ArN + N + +
Aryl diazonium ions are stable in solution only near or below room temperature,
and this also limits the range of compounds that can be successfully substituted by
diazonium ions.
Kinetic investigations revealed second-order kinetic behavior for substitution by
diazonium ions in a number of instances. In the case of phenols, it is the conjugate
base that undergoes substitution. 114 This finding is entirely reasonable, since the depro-
tonated oxy group is a better electron donor than the neutral hydroxy substituent. The
reactivity of the diazonium ion depends on the substituent groups that are present.
Reactivity is increased by EWG and decreased by ERG. 115
An unusual feature of the mechanism for diazonium coupling is that in some
cases proton loss can be demonstrated to be the rate-determining step. This feature is
revealed in two ways. First, diazonium couplings of several naphthalenesulfonate ions
exhibit primary isotope effects in the range 4–6 when deuterium is present at the site
of substitution, clearly indicating that cleavage of the C−H bond is rate determining.
Second, these reactions can also be shown to be general base catalyzed. This, too,
implies that proton removal is rate determining. 116
ArN
NAr
SO H HO S N H S N
3
3
O – O HO 3 OH
+ ArN + N
S
HO S HO S
HO 3
3
3
Because of the limited range of aromatic compounds that react with diazonium
ions, selectivity data comparable to those discussed for other electrophilic substitu-
tions are not available. Diazotization, since it involves a weak electrophile, would be
expected to reveal high substrate and position selectivity.
9.4.7. Substitution of Groups Other than Hydrogen
The general mechanism for EAS suggests that groups other than hydrogen could
be displaced, provided that the electrophile attacked at the substituted carbon. Substi-
tution at a site already having a substituent is called ipso substitution and has been
observed in a number of circumstances. The ease of removal of a substituent depends
on its ability to accommodate a positive charge. This factor determines whether the
114 R. Wistar and P. D. Bartlett, J. Am. Chem. Soc., 63, 413 (1941).
115 A. F. Hegarty, in The Chemistry of the Diazonium and Diazo Groups, S. Patai, ed., John Wiley & Sons,
New York, 1978, Chap. 12; H. Mayr, M. Hartnagel, and K. Grimm, Liebigs Ann., 55 (1997).
116
H. Zollinger, Azo and Diazo Chemistry, transl. H. E. Nursten, Interscience, New York, 1961, Chap. 10;
H. Zollinger, Adv. Phys. Org. Chem., 2, 163 (1964); H. Zollinger, Helv. Chim. Acta, 38, 1597 (1955).