Page 1085 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1085
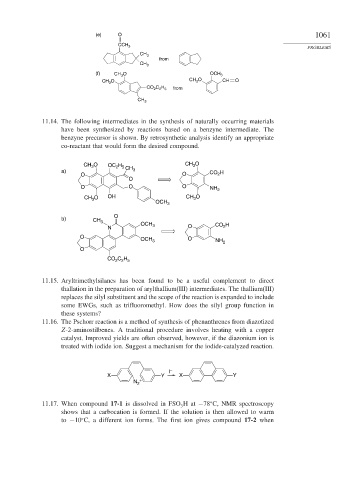
(e) O 1061
CCH 3
PROBLEMS
CH 3
from
CH 3
(f) CH 3 O OCH 3
CH 3 O CH 3 O CH O
CO 2 C 2 H 5 from
CH 3
11.14. The following intermediates in the synthesis of naturally occurring materials
have been synthesized by reactions based on a benzyne intermediate. The
benzyne precursor is shown. By retrosynthetic analysis identify an appropriate
co-reactant that would form the desired compound.
CH 3 O OC H CH CH 3 O
2 5
a) 3 H
O O CO 2
O
O O O NH 2
CH O OH CH 3 O
3
OCH 3
O
b) CH 3
N OCH 3 O CO 2 H
O
OCH 3 O NH 2
O
CO C H
2 2 5
11.15. Aryltrimethylsilanes has been found to be a useful complement to direct
thallation in the preparation of arylthallium(III) intermediates. The thallium(III)
replaces the silyl substituent and the scope of the reaction is expanded to include
some EWGs, such as trifluoromethyl. How does the silyl group function in
these systems?
11.16. The Pschorr reaction is a method of synthesis of phenanthrenes from diazotized
Z-2-aminostilbenes. A traditional procedure involves heating with a copper
catalyst. Improved yields are often observed, however, if the diazonium ion is
treated with iodide ion. Suggest a mechanism for the iodide-catalyzed reaction.
I –
X Y X Y
N 2 +
11.17. When compound 17-1 is dissolved in FSO Hat −78 C, NMR spectroscopy
3
shows that a carbocation is formed. If the solution is then allowed to warm
to −10 C, a different ion forms. The first ion gives compound 17-2 when