Page 1082 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1082
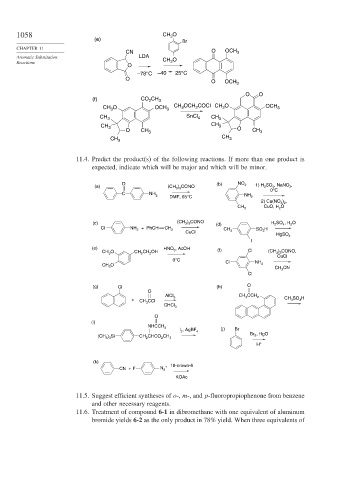
1058 CH 3 O
(e)
Br
CHAPTER 11
CN O OCH 3
Aromatic Substitution LDA CH O
Reactions 3
O
–78°C –40 25°C
O
O OCH 3
O O
(f) CO CH 3
2
CH O OCH 3 CH OCH COCl CH O OCH 3
3
2
3
3
CH 3 SnCl 4 CH 3
CH 3 CH 3
O CH 3 O CH 3
CH 3 CH 3
11.4. Predict the product(s) of the following reactions. If more than one product is
expected, indicate which will be major and which will be minor.
O (b) NO 2
(a) (CH 3 ) 3 CONO 1) H 2 SO 4 , NaNO 2 ,
0°C
C NH 2
DMF, 65°C NH 2
2) Cu(NO 3 ) 2 ,
CuO, H 2 O
CH 3
(c) (CH 3 ) 3 CONO (d) H 2 SO 4 , H 2 O
Cl NH 2 + PhCH CH 2 SO 3 H
CuCl CH 3
HgSO 4
I
(e) HNO 3 , AcOH (f)
CH 3 O CH 2 CH 2 OH Cl (CH 3 ) 3 CONO,
CuCl
0°C
CH 3 O Cl NH 2
CH 3 CN
Cl
(g) Cl (h) O
O
AlCl 3 CH 3 CCH 2 CH 3 SO 3 H
+ CH 3 CCl
CHCl 3
O
(i)
NHCCH 3
(j) Br
I 2 , AgBF 4
Br 2 , HgO
(CH 3 ) 3 Si CH 2 CHCO 2 CH 3
H +
(k)
+ 18-crown-6
CN + F N 2
KOAc
11.5. Suggest efficient syntheses of o-, m-, and p-fluoropropiophenone from benzene
and other necessary reagents.
11.6. Treatment of compound 6-1 in dibromethane with one equivalent of aluminum
bromide yields 6-2 as the only product in 78% yield. When three equivalents of