Page 1079 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1079
S RN 1 substitution include ketone enolates, 183 ester enolates, 184 amide enolates, 185 1055
2,4-pentanedione dianion, 186 pentadienyl and indenyl carbanions, 187 phenolates, 188
diethyl phosphite anion, 189 phosphides, 190 and thiolates. 191 The reactions are frequently SECTION 11.4
initiated by light, which promotes the initiating electron transfer. As for other radical Aromatic Substitution
Reactions Involving
chain processes, the reaction is sensitive to substances that can intercept the propagation Radical Intermediates
intermediates.
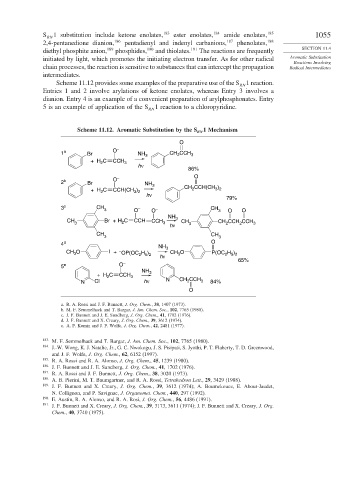
Scheme 11.12 provides some examples of the preparative use of the S 1 reaction.
RN
Entries 1 and 2 involve arylations of ketone enolates, whereas Entry 3 involves a
dianion. Entry 4 is an example of a convenient preparation of arylphosphonates. Entry
5 is an example of application of the S 1 reaction to a chloropyridine.
RN
Scheme 11.12. Aromatic Substitution by the S RN 1 Mechanism
O
1 a Br O – NH 3 CH CCH 3
2
+ H C CCH 3
2
hν
86%
O
–
2 b Br O NH 3
+ H C CCH(CH ) CH 2 CCH(CH 3 ) 2
2
3 2
hν
79%
3 c CH CH
3 O – O – 3 O O
NH 3
CH Br + H C CCH CCH CH CH CCH CCH
3 2 3 hν 3 2 2 3
CH CH
3 3
4 d NH O
CH O I + – OP(OC H ) hν 3 CH O P(OC H )
3
3
2 5 2
2 5 2
5 e O – 65%
NH
+ H C CCH 3 3
2
2
N Cl hν N CH CCH 3 84%
O
a. R. A. Rossi and J. F. Bunnett, J. Org. Chem., 38, 1407 (1973).
b. M. F. Semmelhack and T. Bargar, J. Am. Chem. Soc., 102, 7765 (1980).
c. J. F. Bunnett and J. E. Sundberg, J. Org. Chem., 41, 1702 (1976).
d. J. F. Bunnett and X. Creary, J. Org. Chem., 39, 3612 (1974).
e. A. P. Komin and J. F. Wolfe, J. Org. Chem., 42, 2481 (1977).
183
M. F. Semmelhack and T. Bargar, J. Am. Chem. Soc., 102, 7765 (1980).
184 J.-W. Wong, K. J. Natalie, Jr., G. C. Nwokogu, J. S. Pisipati, S. Jyothi, P. T. Flaherty, T. D. Greenwood,
and J. F. Wolfe, J. Org. Chem., 62, 6152 (1997).
185 R. A. Rossi and R. A. Alonso, J. Org. Chem., 45, 1239 (1980).
186
J. F. Bunnett and J. E. Sundberg, J. Org. Chem., 41, 1702 (1976).
187 R. A. Rossi and J. F. Bunnett, J. Org. Chem., 38, 3020 (1973).
188 A. B. Pierini, M. T. Baumgartner, and R. A. Rossi, Tetrahedron Lett., 29, 3429 (1988).
189
J. F. Bunnett and X. Creary, J. Org. Chem., 39, 3612 (1974); A. Boumekouez, E. About-Jaudet,
N. Collignon, and P. Savignac, J. Organomet. Chem., 440, 297 (1992).
190 E. Austin, R. A. Alonso, and R. A. Rosi, J. Org. Chem., 56, 4486 (1991).
191
J. F. Bunnett and X. Creary, J. Org. Chem., 39, 3173, 3611 (1974); J. F. Bunnett and X. Creary, J. Org.
Chem., 40, 3740 (1975).