Page 1074 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1074
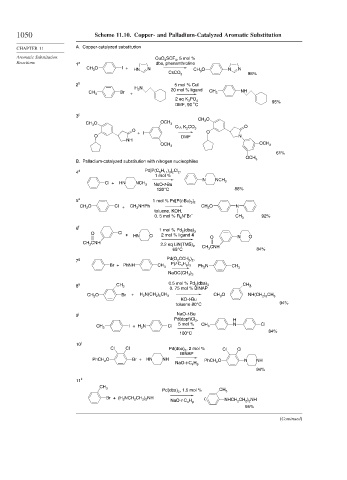
1050 Scheme 11.10. Copper- and Palladium-Catalyzed Aromatic Substitution
CHAPTER 11 A. Copper-catalyzed substitution
Aromatic Substitution CuO 3 SCF 3 , 5 mol %
Reactions 1 a dba, phenanthroline
CH 3 O I + HN N CH 3 O N N
CsCO 3 96%
2 b 5 mol % CuI
H 2 N 20 mol % ligand
Br CH 3 NH
CH 3 +
2 eq K 3 PO 4 95%
DMF, 90 °C
3 c
CH 3 O
CH 3 O OCH 3
O
Cu, K 2 CO 3
O O
O + I DMF N
NH
OCH 3
OCH 3
61%
OCH 3
B. Palladium-catalyzed substitution with nitrogen nucleophiles
4 d Pd[P(C 6 H 11 ) 3 ] 2 Cl 2 ,
1 mol %
N NCH 3
Cl + HN NCH 3 NaO-t-Bu
120°C 88%
5 e 1 mol % Pd[P(t-Bu) 3 ] 2
CH 3 O Cl + CH 3 NHPh CH 3 O N
toluene, KOH,
+
0. 5 mol % R 4 N Br – CH 3 92%
6 f 1 mol % Pd 2 (dba) 3
O Cl + HN O 2 mol % ligand 4 O N O
CH 3 CNH
2.2 eq LiN(TMS) 2 CH 3 CNH
65°C 84%
7 g Pd(O 2 CCH 3 ) 2 ,
Br + PhNH CH 3 P(t-C 4 H 9 ) 3 Ph 2 N CH 3
NaOC(CH 3 ) 3
8 h CH 3 0.5 mol % Pd 2 (dba) 3 CH 3
0. 75 mol % BINAP
CH 3 O Br + H 2 N(CH 2 ) 5 CH 3 CH 3 O NH(CH 2 ) 5 CH 3
KO-t-Bu
toluene 80°C 94%
i NaO-t-Bu
9
Pd(dppf)Cl 2 , H
5 mol % N Cl
I + H 2 N Cl CH 3
CH 3
84%
100°C
10 j
Cl Cl Pd(dba) 2 , 2 mol % Cl Cl
BINAP
PhCH 2 O Br + HN NH PhCH 2 O N NH
NaO-t-C 4 H 9
94%
11 k
CH 3
Pd(dba) 2 , 1.5 mol % CH 3
Br + (H 2 NCH 2 CH 2 ) 2 NH ( NHCH 2 CH 2 ) 2 NH
NaO-t-C 4 H 9
95%
(Continued)