Page 1072 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1072
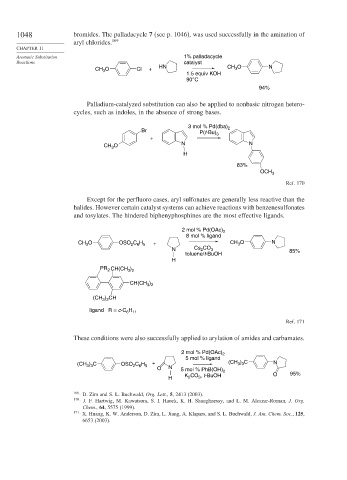
1048 bromides. The palladacycle 7 (see p. 1046), was used successfully in the amination of
aryl chlorides. 169
CHAPTER 11
Aromatic Substitution 1% palladacycle
Reactions catalyst
CH O Cl + HN CH O N
3
3
1.5 equiv KOH
90°C
94%
Palladium-catalyzed substitution can also be applied to nonbasic nitrogen hetero-
cycles, such as indoles, in the absence of strong bases.
3 mol % Pd(dba)
Br P(t-Bu) 3 2
+
CH O N N
3
H
83%
OCH 3
Ref. 170
Except for the perfluoro cases, aryl sulfonates are generally less reactive than the
halides. However certain catalyst systems can achieve reactions with benzenesulfonates
and tosylates. The hindered biphenyphosphines are the most effective ligands.
2 mol % Pd(OAc) 2
8 mol % ligand
CH O OSO C H + CH O N
3
3
2 6 5
N Cs 2 CO 3 85%
toluene/t-BuOH
H
PR 2 CH(CH 3 ) 2
CH(CH )
3 2
(CH ) CH
3 2
H
ligand R = c-C 6 11
Ref. 171
These conditions were also successfully applied to arylation of amides and carbamates.
2 mol % Pd(OAc) 2
5 mol % ligand
(CH ) C OSO C H + (CH ) C N
3 3
2 6 5
3 3
O N
5 mol % PhB(OH) 2
K CO , t-BuOH O 95%
H 2 3
169
D. Zim and S. L. Buchwald, Org. Lett., 5, 2413 (2003).
170 J. F. Hartwig, M. Kawatsura, S. I. Hauck, K. H. Shaughnessy, and L. M. Alcazar-Roman, J. Org.
Chem., 64, 5575 (1999).
171
X. Huang, K. W. Anderson, D. Zim, L. Jiang, A. Klapars, and S. L. Buchwald, J. Am. Chem. Soc., 125,
6653 (2003).