Page 1078 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1078
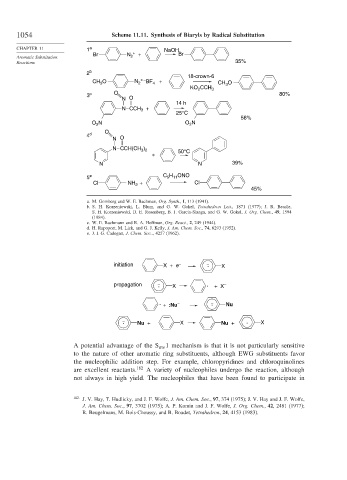
1054 Scheme 11.11. Synthesis of Biaryls by Radical Substitution
CHAPTER 11 1 a NaOH
Br N + + Br
Aromatic Substitution 2
Reactions 35%
2 b
18-crown-6
CH 3 O N 2 + – BF + CH O
4
3
KO CCH 3
2
3 c O 80%
N O
14 h
N CCH 3 +
25°C
56%
O 2 N O 2 N
O
4 d
N O
)
N CCH(CH 3 2 50°C
+
N N 39%
5 11
5 e C H ONO
Cl NH + Cl
2
45%
a. M. Gomberg and W. E. Bachman, Org. Synth., I, 113 (1941).
b. S. H. Korzeniowski, L. Blum, and G. W. Gokel, Tetrahedron Lett., 1871 (1977); J. R. Beadle,
S. H. Korzeniowski, D. E. Rosenberg, B. J. Garcia-Slanga, and G. W. Gokel, J. Org. Chem., 49, 1594
(1984).
c. W. E. Bachmann and R. A. Hoffman, Org. React., 2, 249 (1944).
d. H. Rapoport, M. Lick, and G. J. Kelly, J. Am. Chem. Soc., 74, 6293 (1952).
e. J. I. G. Cadogan, J. Chem. Soc.., 4257 (1962).
–
initiation X + e _ . X
propagation _ . X . + X
–
. + :Nu – _ . Nu
_ _
. Nu + X Nu + . X
A potential advantage of the S 1 mechanism is that it is not particularly sensitive
RN
to the nature of other aromatic ring substituents, although EWG substituents favor
the nucleophilic addition step. For example, chloropyridines and chloroquinolines
are excellent reactants. 182 A variety of nucleophiles undergo the reaction, although
not always in high yield. The nucleophiles that have been found to participate in
182
J. V. Hay, T. Hudlicky, and J. F. Wolfe, J. Am. Chem. Soc., 97, 374 (1975); J. V. Hay and J. F. Wolfe,
J. Am. Chem. Soc., 97, 3702 (1975); A. P. Komin and J. F. Wolfe, J. Org. Chem., 42, 2481 (1977);
R. Beugelmans, M. Bois-Choussy, and B. Boudet, Tetrahedron, 24, 4153 (1983).