Page 1083 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1083
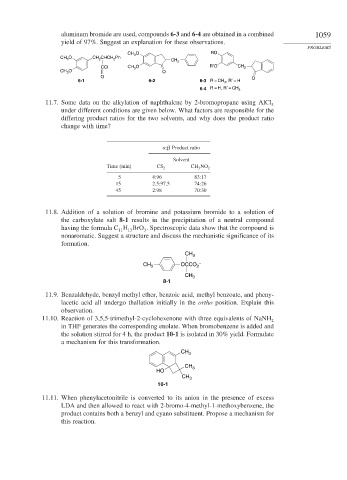
aluminum bromide are used, compounds 6-3 and 6-4 are obtained in a combined 1059
yield of 97%. Suggest an explanation for these observations.
PROBLEMS
CH 3 O RO
CH 3 O CH 2 CHCH 2 Ph
CH 2
CCl CH 3 O R′O CH 2
CH 3 O O
O O
6-1 6-2 6-3 R = CH 3 , R′ = H
6-4 R = H, R′ = CH 3
11.7. Some data on the alkylation of naphthalene by 2-bromopropane using AlCl 3
under different conditions are given below. What factors are responsible for the
differing product ratios for the two solvents, and why does the product ratio
change with time?
: Product ratio
Solvent
Time (min) CS 2 CH 3 NO 2
5 4:96 83:17
15 2.5:97.5 74:26
45 2:98 70:30
11.8. Addition of a solution of bromine and potassium bromide to a solution of
the carboxylate salt 8-1 results in the precipitation of a neutral compound
having the formula C H BrO . Spectroscopic data show that the compound is
11
13
3
nonaromatic. Suggest a structure and discuss the mechanistic significance of its
formation.
CH 3
CH 3 OCCO 2 –
CH 3
8-1
11.9. Benzaldehyde, benzyl methyl ether, benzoic acid, methyl benzoate, and pheny-
lacetic acid all undergo thallation initially in the ortho position. Explain this
observation.
11.10. Reaction of 3,5,5-trimethyl-2-cyclohexenone with three equivalents of NaNH 2
in THF generates the corresponding enolate. When bromobenzene is added and
the solution stirred for 4 h, the product 10-1 is isolated in 30% yield. Formulate
a mechanism for this transformation.
CH 3
CH 3
HO
CH 3
10-1
11.11. When phenylacetonitrile is converted to its anion in the presence of excess
LDA and then allowed to react with 2-bromo-4-methyl-1-methoxybenzene, the
product contains both a benzyl and cyano substituent. Propose a mechanism for
this reaction.