Page 1086 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1086
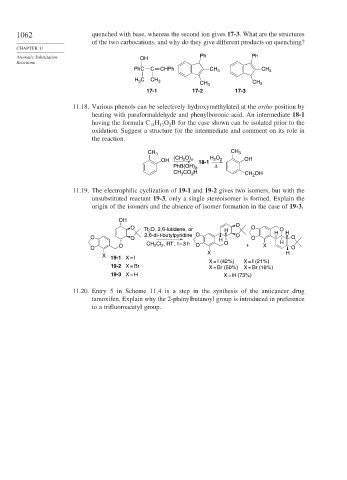
1062 quenched with base, whereas the second ion gives 17-3. What are the structures
of the two carbocations, and why do they give different products on quenching?
CHAPTER 11
Aromatic Substitution OH Ph Ph
Reactions
PhC C CHPh CH 3 CH 3
H C CH 3 CH 3 CH 3
3
17-1 17-2 17-3
11.18. Various phenols can be selectively hydroxymethylated at the ortho position by
heating with paraformaldehyde and phenylboronic acid. An intermediate 18-1
having the formula C H O B for the case shown can be isolated prior to the
13
2
14
oxidation. Suggest a structure for the intermediate and comment on its role in
the reaction.
CH 3 CH 3
(CH O) H O
OH 2 n 18-1 2 2 OH
PhB(OH) , Δ
2
CH CO H CH 2 OH
2
3
11.19. The electrophilic cyclization of 19-1 and 19-2 gives two isomers, but with the
unsubstituted reactant 19-3, only a single stereoisomer is formed. Explain the
origin of the isomers and the absence of isomer formation in the case of 19-3.
OH
O
O Tf 2 O, 2,6-lutidene, or H O O
2,6-di-t-butylpyridine O O H H
O O H O O
CH 2 Cl 2 , RT, 1– 3 h O H
O O + X
O O
X H
X 19-1 X = I
X = I (42%) X = I (21%)
19-2 X = Br X = Br (50%) X = Br (16%)
19-3 X = H X = H (73%)
11.20. Entry 5 in Scheme 11.4 is a step in the synthesis of the anticancer drug
tamoxifen. Explain why the 2-phenylbutanoyl group is introduced in preference
to a trifluoroacetyl group.