Page 1096 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1096
1072 an example of the use of a water-soluble carbodiimide as the activating reagent. The
modified carbodiimide facilitates product purification by providing for easy removal
CHAPTER 12 of the urea by-product. Entries 5 and 6 are examples of the Swern procedure. Entry 7
Oxidations uses TFAA as the electrophile. Entry 8, which uses the inexpensive reagent P O as
2 5
the electrophile, was conducted on a 60-g scale.
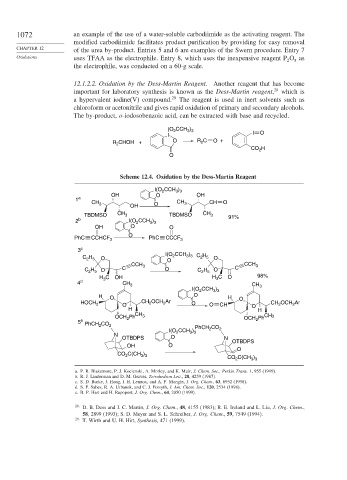
12.1.2.2. Oxidation by the Dess-Martin Reagent. Another reagent that has become
important for laboratory synthesis is known as the Dess-Martin reagent, 28 which is
a hypervalent iodine(V) compound. 29 The reagent is used in inert solvents such as
chloroform or acetonitrile and gives rapid oxidation of primary and secondary alcohols.
The by-product, o-iodosobenzoic acid, can be extracted with base and recycled.
(O CCH )
3 3
2
I I O
R CHOH + O R 2 C O +
2
CO H
2
O
Scheme 12.4. Oxidation by the Dess-Martin Reagent
I(O CCH )
2
3 3
OH O OH
1 a
CH CH 3 CH O
3
OH O
TBDMSO CH 3 TBDMSO CH 3 91%
2 b I(O CCH 3 ) 3
2
OH O O
PhC CCHCF 3 O PhC CCCF 3
3 c
I(O CCH )
2 5
C H O O 2 3 3 C H O
2 5
CCH 3 CCH 3
C H O C O C H O C
2 5
2 5
H C OH H C O 98%
3
3
4 d CH 3 CH
I(O CCH ) 3
3 3
2
H O O H O
HOCH 2 O CH OCH Ar O O CH CH 2 OCH Ar
2
2
2
H O H
CH
Ph 3 CH3
OCH 2 OCH 2 Ph
5 e PhCH CO
2
2
PhCH 2 CO 2
I(O CCH )
2
3 3
N
OTBDPS O N
OTBDPS
OH O
O
CO 2 C(CH 3 ) 3
CO C(CH 3 ) 3
2
a. P. R. Blakemore, P. J. Kocienski, A. Morley, and K. Muir, J. Chem. Soc., Perkin Trans. 1, 955 (1999).
b. R. J. Linderman and D. M. Graves, Tetrahedron Lett., 28, 4259 (1987).
c. S. D. Burke, J. Hong, J. R. Lennox, and A. P. Mongin, J. Org. Chem., 63, 6952 (1998).
d. S. F. Sabes, R. A. Urbanek, and C. J. Forsyth, J. Am. Chem. Soc., 120, 2534 (1998).
e. B. P. Hart and H. Rapoport, J. Org. Chem., 64, 2050 (1999).
28 D. B. Dess and J. C. Martin, J. Org. Chem., 48, 4155 (1983); R. E. Ireland and L. Liu, J. Org. Chem.,
58, 2899 (1993); S. D. Meyer and S. L. Schreiber, J. Org. Chem., 59, 7549 (1994).
29
T. Wirth and U. H. Hirt, Synthesis, 471 (1999).