Page 1101 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1101
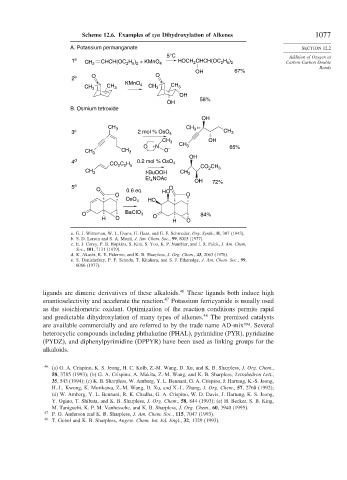
Scheme 12.6. Examples of syn Dihydroxylation of Alkenes 1077
A. Potassium permanganate SECTION 12.2
5°C Addition of Oxygen at
1 a CH 2 CHCH(OC H ) + KMnO 4 HOCH CHCH(OC H ) Carbon-Carbon Double
2
2 5 2
2 5 2
Bonds
OH 67%
2 b O O
KMnO
CH 4 CH CH
CH 3 3 3 3
OH
58%
OH
B. Osmium tetroxide
OH
CH 3 CH 3
3 c 2 mol % OsO 4 CH 3
OH
CH 3
O +N CH 3 65%
CH 3 CH 3 O –
OH
4 d CO C H 0.2 mol % OsO 4 CO CH
2 2 5
CH 3 t-BuOOH CH 3 2 3
Et NOAc OH 72%
4
5 e O
O 0.6 eq. HO
O O
OsO 4 HO
O BaClO 3 O 84%
H O
H O
a. E. J. Witzeman, W. L. Evans, H. Haas, and E. F. Schroeder, Org. Synth., II, 307 (1943).
b. S. D. Larsen and S. A. Monti, J. Am. Chem. Soc., 99, 8015 (1977).
c. E. J. Corey, P. B. Hopkins, S. Kim, S. Yoo, K. P. Nambiar, and J. R. Falck, J. Am. Chem.
Soc., 101, 7131 (1979).
d. K. Akashi, R. E. Palermo, and K. B. Sharpless, J. Org. Chem., 43, 2063 (1978).
e. S. Danishefsky, P. F. Schuda, T. Kitahara, and S. J. Etheredge, J. Am. Chem. Soc., 99,
6066 (1977).
ligands are dimeric derivatives of these alkaloids. 46 These ligands both induce high
47
enantioselectivity and accelerate the reaction. Potassium ferricyanide is usually used
as the stoichiometric oxidant. Optimization of the reaction conditions permits rapid
and predictable dihydroxylation of many types of alkenes. 48 The premixed catalysts
are available commercially and are referred to by the trade name AD-mix™. Several
heterocyclic compounds including phthalazine (PHAL), pyrimidine (PYR), pyridazine
(PYDZ), and diphenylpyrimidine (DPPYR) have been used as linking groups for the
alkaloids.
46
(a) G. A. Crispino, K. S. Jeong, H. C. Kolb, Z.-M. Wang, D. Xu, and K. B. Sharpless, J. Org. Chem.,
58, 3785 (1993); (b) G. A. Crispino, A. Makita, Z.-M. Wang, and K. B. Sharpless, Tetrahedron Lett.,
35, 543 (1994); (c) K. B. Sharpless, W. Amberg, Y. L. Bennani, G. A. Crispino, J. Hartung, K.-S. Jeong,
H.-L. Kwong, K. Morikawa, Z.-M. Wang, D. Xu, and X.-L. Zhang, J. Org. Chem., 57, 2768 (1992);
(d) W. Amberg, Y. L. Bennani, R. K. Chadha, G. A. Crispino, W. D. Davis, J. Hartung, K. S. Jeong,
Y. Ogino, T. Shibata, and K. B. Sharpless, J. Org. Chem., 58, 844 (1993); (e) H. Becker, S. B. King,
M. Taniguchi, K. P. M. Vanhessche, and K. B. Sharpless, J. Org. Chem., 60, 3940 (1995).
47 P. G. Anderson and K. B. Sharpless, J. Am. Chem. Soc., 115, 7047 (1993).
48
T. Gobel and K. B. Sharpless, Angew. Chem. Int. Ed. Engl., 32, 1329 (1993).