Page 1097 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1097
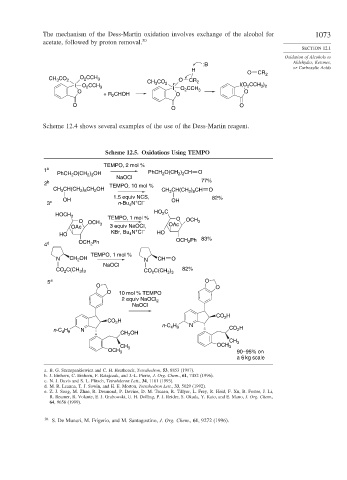
The mechanism of the Dess-Martin oxidation involves exchange of the alcohol for 1073
acetate, followed by proton removal. 30
SECTION 12.1
Oxidation of Alcohols to
Aldehydes, Ketones,
:B
H or Carboxylic Acids
O CR 2
CH CO 2 O CCH 3 CH CO O CR
2
3
I O CCH 3 3 2 I O CCH 2 I(O 2 CCH )
3 2
2
O 2 3 O
+ R 2 CHOH O
O O
O
Scheme 12.4 shows several examples of the use of the Dess-Martin reagent.
Scheme 12.5. Oxidations Using TEMPO
TEMPO, 2 mol %
1 a PhCH O(CH ) CH O
PhCH 2 O(CH ) OH 2 2 2
2 3
NaOCl 77%
2 b TEMPO, 10 mol %
CH(CH ) CH OH
CH 3 2 8 2 CH CH(CH ) CH O
2 8
3
1.5 equiv NCS, 82%
OH OH
+
3 c n-Bu 4 N Cl –
HO C
HOCH 2
2
TEMPO, 1 mol % O
O OCH OCH 3
OAc 3 3 equiv NaOCl, OAc
+
KBr, Bu N Cl – HO
HO 4 83%
2
4 d OCH Ph OCH Ph
2
TEMPO, 1 mol %
N CH OH N CH O
2
NaOCl
C(CH ) 82%
CO 2 3 3 CO C(CH )
2
3 3
5 e O
O O
O 10 mol % TEMPO
2 equiv NaOCl 2
NaOCl
CO H
2
CO H
2
n-C H N
4 9
n-C H N CH 2 OH CO H
2
4 9
CH 3
OCH 3
CH 3
OCH 3 90–95% on
a 6 kg scale
a. B. G. Szczepankiewicz and C. H. Heathcock, Tetrahedron, 53, 8853 (1997).
b. J. Einhorn, C. Einhorn, F. Ratajczak, and J.-L. Pierre, J. Org. Chem., 61, 7452 (1996).
c. N. J. Davis and S. L. Flitsch, Tetrahderon Lett., 34, 1181 (1993).
d. M. R. Leanna, T. J. Sowin, and H. E. Morton, Tetrahedron Lett., 33, 5029 (1992).
e. Z. J. Song, M. Zhao, R. Desmond, P. Devine, D. M. Tscaen, R. Tillyer, L. Frey, R. Heid, F. Xu, B. Foster, J. Li,
R. Reamer, R. Volante, E. J. Grabowski, U. H. Dolling, P. J. Reider, S. Okada, Y. Kato, and E. Mano, J. Org. Chem.,
64, 9658 (1999).
30
S. De Munari, M. Frigerio, and M. Santagostino, J. Org. Chem., 61, 9272 (1996).