Page 1102 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1102
1078
N Ph N
N N N N
CHAPTER 12 O O
O O
H
Oxidations CH 3 O N N
CH 3 O OCH 3 OCH 3
Ph N
N N NN
(DHQ) 2 -PHAL (DHQD) 2 -DPPYR
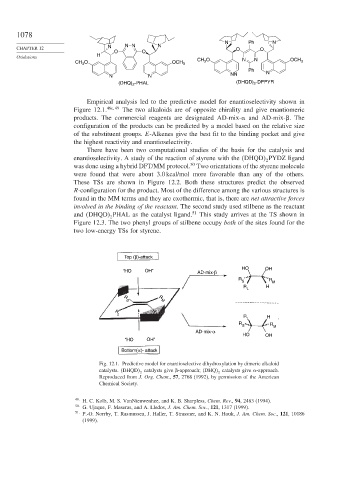
Empirical analysis led to the predictive model for enantioselectivity shown in
Figure 12.1. 46c 49 The two alkaloids are of opposite chirality and give enantiomeric
products. The commercial reagents are designated AD-mix- and AD-mix-
. The
configuration of the products can be predicted by a model based on the relative size
of the substituent groups. E-Alkenes give the best fit to the binding pocket and give
the highest reactivity and enantioselectivity.
There have been two computational studies of the basis for the catalysis and
enantioselectivity. A study of the reaction of styrene with the DHQD PYDZ ligand
2
50
was done using a hybrid DFT/MM protocol. Two orientations of the styrene molecule
were found that were about 3.0 kcal/mol more favorable than any of the others.
These TSs are shown in Figure 12.2. Both these structures predict the observed
R-configuration for the product. Most of the difference among the various structures is
found in the MM terms and they are exothermic, that is, there are net attractive forces
involved in the binding of the reactant. The second study used stilbene as the reactant
and DHQD PHAL as the catalyst ligand. 51 This study arrives at the TS shown in
2
Figure 12.3. The two phenyl groups of stilbene occupy both of the sites found for the
two low-energy TSs for styrene.
Top (β)-attack
HO OH
“HO OH” AD-mix-β
R R
S M
R H
L
R R
S M
R H
L
R L H
R R
S M
AD-mix-α
HO OH
“HO OH”
Bottom(α)- attack
Fig. 12.1. Predictive model for enantioselective dihydroxylation by dimeric alkaloid
catalysts. DHQD catalysts give
-approach; DHQ catalysts give -approach.
2
2
Reproduced from J. Org. Chem., 57, 2768 (1992), by permission of the American
Chemical Society.
49 H. C. Kolb, M. S. VanNieuwenhze, and K. B. Sharpless, Chem. Rev., 94, 2483 (1994).
50 G. Ujaque, F. Maseras, and A. Lledos, J. Am. Chem. Soc., 121, 1317 (1999).
51
P.-O. Norrby, T. Rasmussen, J. Haller, T. Strassner, and K. N. Houk, J. Am. Chem. Soc., 121, 10186
(1999).