Page 1105 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1105
observed lactone. This particular oxidation was also carried out with DHQD -PHAL, 1081
2
which gave the enantiomeric lactone. Entry 4 is an optimized oxidation of stilbene
that was done on a 1-kg scale. Entry 5 is the dihydroxylation of geranyl acetate that SECTION 12.2
shows selectivity for the 6,7-double bond. Entry 6 involves an unsaturated amide and Addition of Oxygen at
Carbon-Carbon Double
required somewhat higher catalyst loading than normal. Entry 7 provided a starting Bonds
material for the enantioselective synthesis of S-ibuprofen. The reaction in Entry 8
was used to prepare the lactone shown (and its enantiomer) as starting materials for
enantioselective synthesis of several natural products. The furan synthesized in Entry 9
was used to prepare a natural material by a route involving eventual oxidation of the
furan ring.
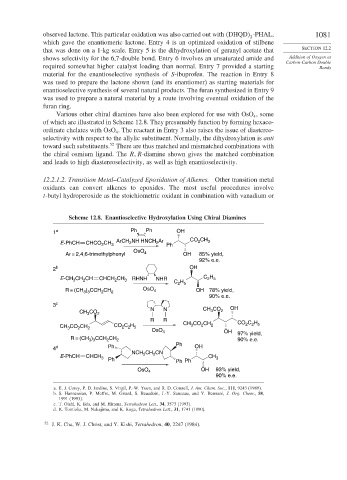
Various other chiral diamines have also been explored for use with OsO , some
4
of which are illustrated in Scheme 12.8. They presumably function by forming hexaco-
ordinate chelates with OsO . The reactant in Entry 3 also raises the issue of diastereo-
4
selectivity with respect to the allylic substituent. Normally, the dihydroxylation is anti
52
toward such substituents. There are thus matched and mismatched combinations with
the chiral osmium ligand. The R R-diamine shown gives the matched combination
and leads to high diastereoselectivity, as well as high enantioselectivity.
12.2.1.2. Transition Metal–Catalyzed Epoxidation of Alkenes. Other transition metal
oxidants can convert alkenes to epoxides. The most useful procedures involve
t-butyl hydroperoxide as the stoichiometric oxidant in combination with vanadium or
Scheme 12.8. Enantioselective Hydroxylation Using Chiral Diamines
1 a Ph Ph OH
2
E-PhCH CHCO 2 CH 3 ArCH NH HNCH Ar Ph CO CH 3
2
2
OsO
Ar = 2,4,6-trimethylphenyl 4 OH 85% yield,
92% e.e.
2 b OH
E-CH CH CH CHCH CH 3 RHNH NHR C H C H
2 5
2
3
2
2 5
R = (CH ) CCH CH 2 OsO 4 OH 78% yield,
3 3
2
90% e.e.
3 c
N N CH CO OH
CH CO 2 3 2
3
R R CO C H
3
2
CH CO CH 2 CO C H CH CO CH 2 2 2 5
2 2 5
3
2
OsO 4 OH 97% yield,
R = (CH ) CCH CH 2 90% e.e.
3 3
2
Ph
4 d Ph OH
NCH CH CN
E-PhCH CHCH 3 Ph 2 2 Ph Ph CH 3
OsO 4 OH 93% yield,
90% e.e.
a. E. J. Corey, P. D. Jardine, S. Virgil, P.-W. Yuen, and R. D. Connell, J. Am. Chem. Soc., 111, 9243 (1989).
b. S. Hannessian, P. Meffre, M. Girard, S. Beaudoin, J.-Y. Sanceau, and Y. Bennani, J. Org. Chem., 58,
1991 (1993).
c. T. Oishi, K. Iida, and M. Hirama, Tetrahedron Lett., 34, 3573 (1993).
d. K. Tomioka, M. Nakajima, and K. Koga, Tetrahedron Lett., 31, 1741 (1990).
52
J. K. Cha, W. J. Christ, and Y. Kishi, Tetrahedron, 40, 2247 (1984).