Page 1103 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1103
1079
SECTION 12.2
Addition of Oxygen at
Carbon-Carbon Double
Bonds
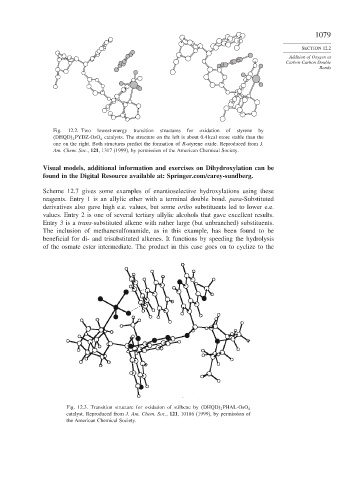
Fig. 12.2. Two lowest-energy transition structures for oxidation of styrene by
DHQD 2 PYDZ-OsO 4 catalysts. The structure on the left is about 0.4 kcal more stable than the
one on the right. Both structures predict the formation of R-styrene oxide. Reproduced from J.
Am. Chem. Soc., 121, 1317 (1999), by permission of the American Chemical Society.
Visual models, additional information and exercises on Dihydroxylation can be
found in the Digital Resource available at: Springer.com/carey-sundberg.
Scheme 12.7 gives some examples of enantioselective hydroxylations using these
reagents. Entry 1 is an allylic ether with a terminal double bond. para-Substituted
derivatives also gave high e.e. values, but some ortho substituents led to lower e.e.
values. Entry 2 is one of several tertiary allylic alcohols that gave excellent results.
Entry 3 is a trans-substituted alkene with rather large (but unbranched) substituents.
The inclusion of methanesulfonamide, as in this example, has been found to be
beneficial for di- and trisubstituted alkenes. It functions by speeding the hydrolysis
of the osmate ester intermediate. The product in this case goes on to cyclize to the
Fig. 12.3. Transition structure for oxidation of stilbene by DHQD 2 PHAL-OsO 4
catalyst. Reproduced from J. Am. Chem. Soc., 121, 10186 (1999), by permission of
the American Chemical Society.