Page 1108 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1108
1084
(a) (b)
CHAPTER 12 1.357Å
Oxidations 1.832Å
1.856Å
2.304Å 1.852Å
1.783Å 1.367Å 1.959Å
1.947Å 2.210Å 2.226Å 2.015Å
67.2° 1.766Å
1.710Å
1.817Å 2.033Å
(c) (d)
O11
O10
O5 O2
O1
O8 O3
O9
O4
O6
(e)
H
R
E R 1
E
O O O
O O
H OR′
Ti R 2
Ti
O O
H H
O H OR′
O
t-Bu H
E
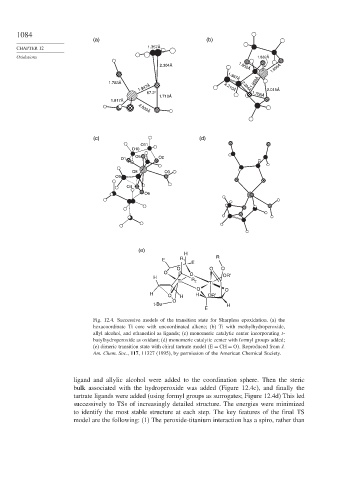
Fig. 12.4. Successive models of the transition state for Sharpless epoxidation. (a) the
hexacoordinate Ti core with uncoordinated alkene; (b) Ti with methylhydroperoxide,
allyl alcohol, and ethanediol as ligands; (c) monomeric catalytic center incorporating t-
butylhydroperoxide as oxidant; (d) monomeric catalytic center with formyl groups added;
(e) dimeric transition state with chiral tartrate model E = CH = O . Reproduced from J.
Am. Chem. Soc., 117, 11327 (1995), by permission of the American Chemical Society.
ligand and allylic alcohol were added to the coordination sphere. Then the steric
bulk associated with the hydroperoxide was added (Figure 12.4c), and finally the
tartrate ligands were added (using formyl groups as surrogates; Figure 12.4d) This led
successively to TSs of increasingly detailed structure. The energies were minimized
to identify the most stable structure at each step. The key features of the final TS
model are the following: (1) The peroxide-titanium interaction has a spiro, rather than