Page 1111 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1111
O-i -Pr 1087
t -Bu
t -Bu O
O i -Pr-O Ti t -Bu SECTION 12.2
i -Pr-O O 1 O i -Pr-O O R 1 O Addition of Oxygen at
Ti R H R 1 H Ti H Carbon-Carbon Double
O O O Bonds
i -Pr-O H * i -Pr-O * H
* R 2 H * R 2
* R 2
CH 3 H C CH 3
3
syn syn
t -Bu O-i -Pr
O t -Bu t -Bu
i -Pr-O O 1 O i -Pr-O O O
Ti R H i -Pr-O Ti Ti R 1 H
O R 1 O
i -Pr-O H O
CH 3 O i -Pr-O *
* R 2 * CH 3 R 2
H * CH
* 3 R 2 H
H anti anti
Pre-Reaction Complexes Transition Structures Product Complexes
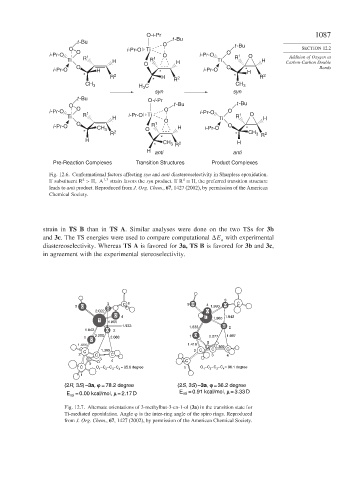
Fig. 12.6. Conformational factors affecting syn and anti diastereoselectivity in Sharpless epoxidation.
4
4
If substituent R > H A 1 3 strain favors the syn product. If R = H, the preferred transition structure
leads to anti product. Reproduced from J. Org. Chem., 67, 1427 (2002), by permission of the American
Chemical Society.
strain in TS B than in TS A. Similar analyses were done on the two TSs for 3b
and 3c. The TS energies were used to compare computational E with experimental
a
diastereoselectivity. Whereas TS A is favored for 3a, TS B is favored for 3b and 3c,
in agreement with the experimental stereoselectivity.
3
3 C 6 5 O C
5 O 4 1.990 O
O
2.022 O
O 4 T 1.843
T 1.965
1.805
1.933 1.833 O 2
1.843 O 2
2.255 1 O 2.277 1.997
1 2.088
O
1.411 1.419 5
C C 1.369 C
C 1.365 2 C
2 C C 3 4
C 3 4 C
5
C O – C – C – C = 35.6 degree 1 O – C – C – C = 96.1 degree
1 2 3 4 1 2 3 4
1
(2R, 3S) –3a, ϕ = 78.2 degree (2S, 3S) –3a, ϕ = 36.2 degree
= 0.00 kcal/mol, μ = 2.17 D E = 0.91 kcal/mol, μ = 3.33 D
E rel rel
Fig. 12.7. Alternate orientations of 3-methylbut-3-en-1-ol (3a) in the transition state for
Ti-mediated epoxidation. Angle is the inter-ring angle of the spiro rings. Reproduced
from J. Org. Chem., 67, 1427 (2002), by permission of the American Chemical Society.