Page 1114 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1114
1090
CHAPTER 12
N N N N N N
Oxidations
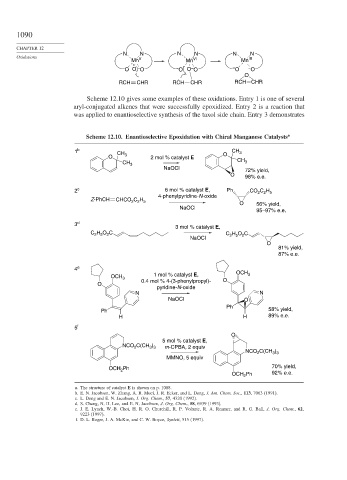
Mn V Mn VI Mn III
– O O – O – O O – O – O – O
O
RCH CHR RCH CHR RCH CHR
Scheme 12.10 gives some examples of these oxidations. Entry 1 is one of several
aryl-conjugated alkenes that were successfully epoxidized. Entry 2 is a reaction that
was applied to enantioselective synthesis of the taxol side chain. Entry 3 demonstrates
Scheme 12.10. Enantioselective Epoxidation with Chiral Manganese Catalysts a
1 b CH O CH 3
O 3 2 mol % catalyst E
CH
CH 3 3
NaOCl 72% yield,
O 98% e.e.
C H
2 c 6 mol % catalyst E, Ph CO 2 2 5
4-phenylpyridine-N-oxide
Z-PhCH CHCO 2 2 5
C H
O 56% yield,
NaOCl
95–97% e.e.
3 d
3 mol % catalyst E,
C H O C C H O C
2
2 5
NaOCl 2 5 2
O
81% yield,
87% e.e.
4 e OCH
OCH 3 1 mol % catalyst E, 3
O 0.4 mol % 4-(3-phenylpropyl)- O
pyridine-N-oxide
N N
NaOCl O
Ph
Ph 58% yield,
H H 89% e.e.
5 f
O
5 mol % catalyst E,
NCO C(CH ) m-CPBA, 2 equiv
2
3 3
C(CH )
NCO 2 3 3
MMNO, 5 equiv
OCH Ph 70% yield,
2
OCH Ph 92% e.e.
2
a. The structure of catalyst E is shown on p. 1088.
b. E. N. Jacobsen, W. Zhang, A. R. Muci, J. R. Ecker, and L. Deng, J. Am. Chem. Soc., 113, 7063 (1991).
c. L. Deng and E. N. Jacobsen, J. Org. Chem., 57, 4320 (1992).
d. S. Chang, N. H. Lee, and E. N. Jacobsen, J. Org. Chem., 58, 6939 (1993).
e. J. E. Lynch, W.-B. Choi, H. R. O. Churchill, R. P. Volante, R. A. Reamer, and R. G. Ball, J. Org. Chem., 62,
9223 (1997).
f. D. L. Boger, J. A. McKie, and C. W. Boyce, Synlett, 515 (1997).