Page 1113 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1113
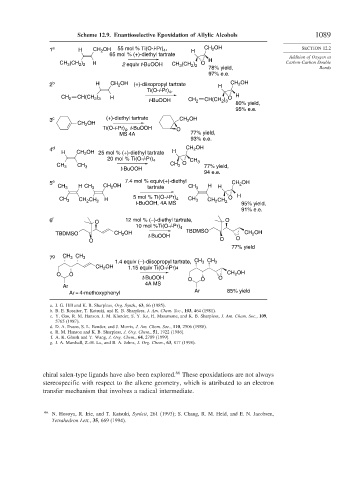
Scheme 12.9. Enantioselective Epoxidation of Allylic Alcohols 1089
1 a H CH 2 OH 55 mol % Ti(O-i-Pr) 4 , H CH OH SECTION 12.2
2
65 mol % (+)-diethyl tartrate Addition of Oxygen at
H
CH (CH ) H 2 equiv t-BuOOH CH 3 (CH ) O Carbon-Carbon Double
3
2 2
2 2
78% yield, Bonds
97% e.e.
2 b H CH 2 OH (+)-diisopropyl tartrate H CH 2 OH
,
Ti(O-i-Pr) 4
CH 2 CH(CH ) H CH CH(CH ) O H
2 3
t-BuOOH
2 3
2
80% yield,
95% e.e.
3 c (+)-diethyl tartrate CH 2 OH
CH OH
2
Ti(O-i-Pr) , t-BuOOH O
4
MS 4A 77% yield,
93% e.e.
4 d H CH 2 OH
H CH OH 25 mol % (+)-diethyl tartrate
2
20 mol % Ti(O-i-Pr) 4 CH 3
CH 3 CH 3 CH 3 O 77% yield,
t-BuOOH
94 e.e.
5 e 7.4 mol % equiv(+)-diethyl CH OH
2
CH 3 H CH 3 CH 2 OH tartrate CH 3 H H
CH 3 CH CH 2 H 5 mol % Ti(O-i-Pr) 4 CH 3 CH CH 2 O H 95% yield,
2
2
t-BuOOH, 4A MS
91% e.e.
6 f O 12 mol % (–)-diethyl tartrate, O
10 mol %Ti(O-i-Pr) 4
TBDMSO CH OH t-BuOOH TBDMSO CH OH
2
2
O O O
77% yield
7 g CH CH 3
3
1.4 equiv (–)-diisopropyl tartrate, CH CH 3
3
CH OH 1.15 equiv Ti(O-i-Pr)4
2
CH OH
O O 2
t-BuOOH O O O
4A MS
Ar
Ar = 4-methoxyphenyl Ar 85% yield
a. J. G. Hill and K. B. Sharpless, Org. Synth., 63, 66 (1985).
b. B. E. Rossiter, T. Katsuki, and K. B. Sharpless, J. Am. Chem. Soc., 103, 464 (1981).
c. Y. Gao, R. M. Hanson. J. M. Klunder, S. Y. Ko, H. Masamune, and K. B. Sharpless, J. Am. Chem. Soc., 109,
5765 (1987).
d. D. A. Evans, S. L. Bender, and J. Morris, J. Am. Chem. Soc., 110, 2506 (1988).
e. R. M. Hanson and K. B. Sharpless, J. Org. Chem., 51, 1922 (1986).
f. A. K. Ghosh and Y. Wang, J. Org. Chem., 64, 2789 (1999).
g. J. A. Marshall, Z.-H. Lu, and B. A. Johns, J. Org. Chem., 63, 817 (1998).
66
chiral salen-type ligands have also been explored. These epoxidations are not always
stereospecific with respect to the alkene geometry, which is attributed to an electron
transfer mechanism that involves a radical intermediate.
66
N. Hosoya, R. Irie, and T. Katsuki, Synlett, 261 (1993); S. Chang, R. M. Heid, and E. N. Jacobsen,
Tetrahedron Lett., 35, 669 (1994).