Page 1107 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1107
E 1083
O RO O E R
R O CH 2 OH SECTION 12.2
CH OH Ti E Ti
2
O
O Addition of Oxygen at
OR Carbon-Carbon Double
E Bonds
O R
E
O R RO O E
E O O
Ti E Ti
RO E O O
O O O OR
Ti E Ti E
O R
O O OR
E ) CO H
) C
(CH 3 3 E E (CH 3 3 2
O
RO O R
Ti E Ti O
O O O
E O
(CH ) C
3 3
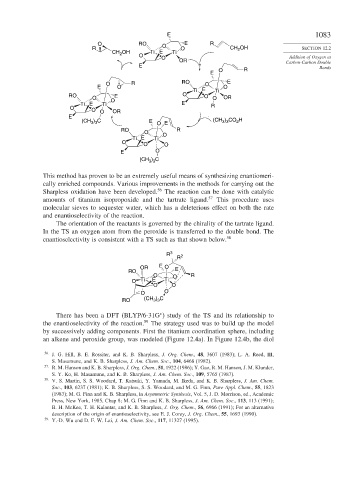
This method has proven to be an extremely useful means of synthesizing enantiomeri-
cally enriched compounds. Various improvements in the methods for carrying out the
Sharpless oxidation have been developed. 56 The reaction can be done with catalytic
amounts of titanium isopropoxide and the tartrate ligand. 57 This procedure uses
molecular sieves to sequester water, which has a deleterious effect on both the rate
and enantioselectivity of the reaction.
The orientation of the reactants is governed by the chirality of the tartrate ligand.
In the TS an oxygen atom from the peroxide is transferred to the double bond. The
enantioselectivity is consistent with a TS such as that shown below. 58
R 3
R 2
OR E O E
RO
O O R
O Ti E Ti
O O
O O
RO (CH ) C
3 3
∗
There has been a DFT (BLYP/6-31G ) study of the TS and its relationship to
the enantioselectivity of the reaction. 59 The strategy used was to build up the model
by successively adding components. First the titanium coordination sphere, including
an alkene and peroxide group, was modeled (Figure 12.4a). In Figure 12.4b, the diol
56
J. G. Hill, B. E. Rossiter, and K. B. Sharpless, J. Org. Chem., 48, 3607 (1983); L. A. Reed, III,
S. Masamune, and K. B. Sharpless, J. Am. Chem. Soc., 104, 6468 (1982).
57
R. M. Hanson and K. B. Sharpless, J. Org. Chem., 51, 1922 (1986); Y. Gao, R. M. Hanson, J. M. Klunder,
S. Y. Ko, H. Masamune, and K. B. Sharpless, J. Am. Chem. Soc., 109, 5765 (1987).
58 V. S. Martin, S. S. Woodard, T. Katsuki, Y. Yamada, M. Ikeda, and K. B. Sharpless, J. Am. Chem.
Soc., 103, 6237 (1981); K. B. Sharpless, S. S. Woodard, and M. G. Finn, Pure Appl. Chem., 55, 1823
(1983); M. G. Finn and K. B. Sharpless, in Asymmetric Synthesis, Vol. 5, J. D. Morrison, ed., Academic
Press, New York, 1985, Chap 8; M. G. Finn and K. B. Sharpless, J. Am. Chem. Soc., 113, 113 (1991);
B. H. McKee, T. H. Kalantar, and K. B. Sharpless, J. Org. Chem., 56, 6966 (1991); For an alternative
description of the origin of enantioselectivity, see E. J. Corey, J. Org. Chem., 55, 1693 (1990).
59
Y.-D. Wu and D. F. W. Lai, J. Am. Chem. Soc., 117, 11327 (1995).