Page 1098 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1098
1074 12.1.2.3. Oxidations Using Oxoammonium Ions. Another oxidation procedure uses
an oxoammonium ion, usually derived from the stable nitroxide tetramethylpiperidine
CHAPTER 12 31
nitroxide, TEMPO, as the active reagent. It is regenerated in a catalytic cycle using
Oxidations hypochlorite ion 32 or NCS 33 as the stoichiometric oxidant. These reactions involve an
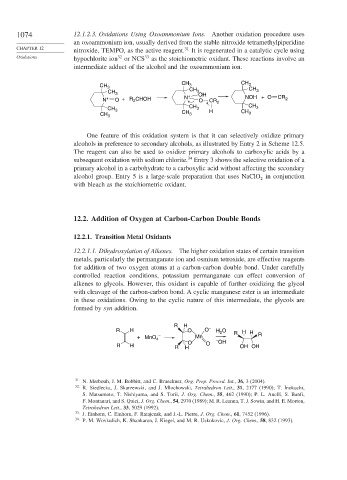
intermediate adduct of the alcohol and the oxoammonium ion.
CH CH
CH 3 3 3
CH 3 CH 3 OH CH 3
N + O +R CHOH N + O CR 2 NOH + O CR 2
2
CH CH
CH 3 3 3
CH 3 CH 3 H CH 3
One feature of this oxidation system is that it can selectively oxidize primary
alcohols in preference to secondary alcohols, as illustrated by Entry 2 in Scheme 12.5.
The reagent can also be used to oxidize primary alcohols to carboxylic acids by a
34
subsequent oxidation with sodium chlorite. Entry 3 shows the selective oxidation of a
primary alcohol in a carbohydrate to a carboxylic acid without affecting the secondary
alcohol group. Entry 5 is a large-scale preparation that uses NaClO in conjunction
2
with bleach as the stoichiometric oxidant.
12.2. Addition of Oxygen at Carbon-Carbon Double Bonds
12.2.1. Transition Metal Oxidants
12.2.1.1. Dihydroxylation of Alkenes. The higher oxidation states of certain transition
metals, particularly the permanganate ion and osmium tetroxide, are effective reagents
for addition of two oxygen atoms at a carbon-carbon double bond. Under carefully
controlled reaction conditions, potassium permanganate can effect conversion of
alkenes to glycols. However, this oxidant is capable of further oxidizing the glycol
with cleavage of the carbon-carbon bond. A cyclic manganese ester is an intermediate
in these oxidations. Owing to the cyclic nature of this intermediate, the glycols are
formed by syn addition.
R H
R H O O – H 2 O R H H
+ MnO 4 – Mn R
O O – OH
R H R H OH OH
31 N. Merbouh, J. M. Bobbitt, and C. Brueckner, Org. Prep. Proced. Int., 36, 3 (2004).
32
R. Siedlecka, J. Skarzewski, and J. Mlochowski, Tetrahedron Lett., 31, 2177 (1990); T. Inokuchi,
S. Matsumoto, T. Nishiyama, and S. Torii, J. Org. Chem., 55, 462 (1990); P. L. Anelli, S. Banfi,
F. Montanari, and S. Quici, J. Org. Chem., 54, 2970 (1989); M. R. Leanna, T. J. Sowin, and H. E. Morton,
Tetrahedron Lett., 33, 5029 (1992).
33 J. Einhorn, C. Einhorn, F. Ratajczak, and J.-L. Pierre, J. Org. Chem., 61, 7452 (1996).
34
P. M. Wovkulich, K. Shankaran, J. Kiegel, and M. R. Uskokovic, J. Org. Chem., 58, 832 (1993).